Alveolar Macrophage Chemokine Secretion Mediates Neutrophilic Lung Injury in Nox2-Deficient Mice
- PMID: 30288635
- PMCID: PMC6775637
- DOI: 10.1007/s10753-018-0883-7
Alveolar Macrophage Chemokine Secretion Mediates Neutrophilic Lung Injury in Nox2-Deficient Mice
Abstract
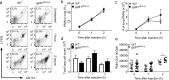
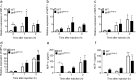
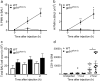
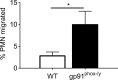
Acute lung injury (ALI), developing as a component of the systemic inflammatory response syndrome (SIRS), leads to significant morbidity and mortality. Reactive oxygen species (ROS), produced in part by the neutrophil NADPH oxidase 2 (Nox2), have been implicated in the pathogenesis of ALI. Previous studies in our laboratory demonstrated the development of pulmonary inflammation in Nox2-deficient (gp91phox-/y) mice that was absent in WT mice in a murine model of SIRS. Given this finding, we hypothesized that Nox2 in a resident cell in the lung, specifically the alveolar macrophage, has an essential anti-inflammatory role. Using a murine model of SIRS, we examined whole-lung digests and bronchoalveolar lavage fluid (BALf) from WT and gp91phox-/y mice. Both genotypes demonstrated neutrophil sequestration in the lung during SIRS, but neutrophil migration into the alveolar space was only present in the gp91phox-/y mice. Macrophage inflammatory protein (MIP)-1α gene expression and protein secretion were higher in whole-lung digest from uninjected gp91phox-/y mice compared to the WT mice. Gene expression of MIP-1α, MCP-1, and MIP-2 was upregulated in alveolar macrophages obtained from gp91phox-/y mice at baseline compared with WT mice. Further, ex vivo analysis of alveolar macrophages, but not bone marrow-derived macrophages or peritoneal macrophages, demonstrated higher gene expression of MIP-1α and MIP-2. Moreover, isolated lung polymorphonuclear neutrophils migrate to BALf obtained from gp91phox-/y mice, further providing evidence of a cell-specific anti-inflammatory role for Nox2 in alveolar macrophages. We speculate that Nox2 represses the development of inflammatory lung injury by modulating chemokine expression by the alveolar macrophage.
Keywords: ARDS; NADPH oxidase 2; Nox2; acute lung injury; alveolar macrophage.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

