Long-term efficacy of novel therapies in moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response
- PMID: 30289198
- PMCID: PMC6587780
- DOI: 10.1111/jdv.15277
Long-term efficacy of novel therapies in moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response
Abstract
Background: Patients with moderate-to-severe psoriasis require long-term treatment, yet few trials compare outcomes beyond a short-term induction period. Quantitative comparisons of long-term outcomes in patients with psoriasis are limited. To our knowledge, no network meta-analysis (NMA) of such data has been performed.
Objective: To compare novel systemic therapies, both biologic and non-biologic, approved for moderate-to-severe psoriasis by conducting a systematic review (SR) and NMA of Psoriasis Area and Severity Index (PASI) outcomes measured at or around 1 year.
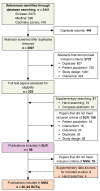
Methods: An SR was conducted to identify studies reporting PASI 75, PASI 90 and PASI 100 responses. Feasibility of an NMA on maintenance phase endpoints was assessed and sources of heterogeneity considered. Data appropriate for analysis were modelled using a Bayesian multinomial likelihood model with probit link. Wherever possible, data corresponding to an intention-to-treat approach with non-responder imputation were used.
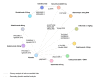
Results: Twenty-four studies reporting outcomes at 40-64 weeks were identified, but heterogeneity in study design allowed synthesis of only 17. Four 52-week randomized controlled trials (RCTs) comprised the primary analysis, which found brodalumab was significantly more efficacious than secukinumab, ustekinumab and etanercept. Secukinumab was also more efficacious than ustekinumab and both outperformed etanercept. In a secondary analysis, evidence from 13 additional studies and 4 further therapies (adalimumab, apremilast, infliximab and ixekizumab) was included by comparing long-term outcomes from active interventions to placebo outcomes extrapolated from induction. Results were consistent with the primary analysis: brodalumab was most effective, followed by ixekizumab and secukinumab, then ustekinumab, infliximab and adalimumab. Etanercept and apremilast had the lowest expected long-term efficacy. Results were similar when studies with low prior exposure to biological therapies were excluded.
Conclusion: Results suggest that brodalumab is associated with a higher likelihood of sustained PASI response, including complete clearance, at week 52 than comparators. Further long-term active-comparator RCT data are required to better assess relative efficacy across therapies.
© 2018 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Figures


References
-
- Goff KL, Karimkhani C, Boyers LN et al The global burden of psoriatic skin disease. Br J Dermatol 2015; 172: 1665–1668. - PubMed
-
- de Korte J, Sprangers MA, Mombers FM, Bos JD. Quality of life in patients with psoriasis: a systematic literature review. J Investig Dermatol Symp Proc 2004; 9: 140–147. - PubMed
-
- Feldman SR, Burudpakdee C, Gala S, Nanavaty M, Mallya UG. The economic burden of psoriasis: a systematic literature review. Expert Rev Pharmacoecon Outcomes Res 2014; 14: 685–705. - PubMed
-
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007; 370: 263–271. - PubMed
-
- van de Kerkhof PC. The relevance of biologics for the treatment of patients with psoriasis. Br J Dermatol 2009; 161: 1213–1214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

