Interventionist versus expectant care for severe pre-eclampsia between 24 and 34 weeks' gestation
- PMID: 30289565
- PMCID: PMC6517196
- DOI: 10.1002/14651858.CD003106.pub3
Interventionist versus expectant care for severe pre-eclampsia between 24 and 34 weeks' gestation
Abstract
Background: Severe pre-eclampsia can cause significant mortality and morbidity for both mother and child, particularly when it occurs remote from term, between 24 and 34 weeks' gestation. The only known cure for this disease is delivery. Some obstetricians advocate early delivery to ensure that the development of serious maternal complications, such as eclampsia (fits) and kidney failure are prevented. Others prefer a more expectant approach, delaying delivery in an attempt to reduce the mortality and morbidity for the child that is associated with being born too early.
Objectives: To evaluate the comparative benefits and risks of a policy of early delivery by induction of labour or by caesarean section, after sufficient time has elapsed to administer corticosteroids, and allow them to take effect; with a policy of delaying delivery (expectant care) for women with severe pre-eclampsia between 24 and 34 weeks' gestation.
Search methods: For this update, we searched Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) on 27 November 2017, and reference lists of retrieved studies.
Selection criteria: Randomised trials comparing the two intervention strategies for women with early onset, severe pre-eclampsia. Trials reported in an abstract were eligible for inclusion, as were cluster-trial designs. We excluded quasi-randomised trials.
Data collection and analysis: Three review authors independently assessed trials for inclusion and risk of bias, extracted data, and checked them for accuracy. We assessed the quality of the evidence for specified outcomes using the GRADE approach.
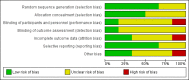
Main results: We included six trials, with a total of 748 women in this review. All trials included women in whom there was no overriding indication for immediate delivery in the fetal or maternal interest. Half of the trials were at low risk of bias for methods of randomisation and allocation concealment; and four trials were at low risk for selective reporting. For most other domains, risk of bias was unclear. There were insufficient data for reliable conclusions about the comparative effects on most outcomes for the mother. Two studies reported on maternal deaths; neither study reported any deaths (two studies; 320 women; low-quality evidence). It was uncertain whether interventionist care reduced eclampsia (risk ratio (RR) 0.98, 95% confidence interval (CI) 0.06 to 15.58; two studies; 359 women) or pulmonary oedema (RR 0.45, 95% CI 0.07 to 3.00; two studies; 415 women), because the quality of the evidence for these outcomes was very low. Evidence from two studies suggested little or no clear difference between the interventionist and expectant care groups for HELLP (haemolysis, elevated liver enzymes, and low platelets) syndrome (RR 1.09, 95% CI 0.62 to 1.91; two studies; 359 women; low-quality evidence). No study reported on stroke. With the addition of data from two studies for this update, there was now evidence to suggest that interventionist care probably made little or no difference to the incidence of caesarean section (average RR 1.01, 95% CI 0.91 to 1.12; six studies; 745 women; Heterogeneity: Tau² = 0.01; I² = 63%).For the baby, there was insufficient evidence to draw reliable conclusions about the effects on perinatal deaths (RR 1.11, 95% CI 0.62 to 1.99; three studies; 343 women; low-quality evidence). Babies whose mothers had been allocated to the interventionist group had more intraventricular haemorrhage (RR 1.94, 95% CI 1.15 to 3.29; two studies; 537 women; moderate-quality evidence), more respiratory distress caused by hyaline membrane disease (RR 2.30, 95% CI 1.39 to 3.81; two studies; 133 women), required more ventilation (RR 1.50, 95% CI 1.11 to 2.02; two studies; 300 women), and were more likely to have a lower gestation at birth (mean difference (MD) -9.91 days, 95% CI -16.37 to -3.45 days; four studies; 425 women; Heterogeneity: Tau² = 31.74; I² = 76%). However, babies whose mothers had been allocated to the interventionist group were no more likely to be admitted to neonatal intensive care (average RR 1.19, 95% CI 0.89 to 1.60; three studies; 400 infants; Heterogeneity: Tau² = 0.05; I² = 84%). Babies born to mothers in the interventionist groups were more likely to have a longer stay in the neonatal intensive care unit (MD 7.38 days, 95% CI -0.45 to 15.20 days; three studies; 400 women; Heterogeneity: Tau² = 40.93, I² = 85%) and were less likely to be small-for-gestational age (RR 0.38, 95% CI 0.24 to 0.61; three studies; 400 women). There were no clear differences between the two strategies for any other outcomes.
Authors' conclusions: This review suggested that an expectant approach to the management of women with severe early onset pre-eclampsia may be associated with decreased morbidity for the baby. However, this evidence was based on data from only six trials. Further large, high-quality trials are needed to confirm or refute these findings, and establish if this approach is safe for the mother.
Conflict of interest statement
David Churchill: None
Lelia Duley: LD has been awarded an NIHR research grant for a programme of work on care at very preterm birth.
Jim G Thornton: Jim Thornton is an author on one of the included studies (Thornton 2004 (GRIT)). However, he was not involved in any assessment, data extraction, or data analysis of this trial.
Mahmoud Moussa: None
Hind SM Ali: None
Kate F Walker: None
Figures























Update of
-
Interventionist versus expectant care for severe pre-eclampsia between 24 and 34 weeks' gestation.Cochrane Database Syst Rev. 2013 Jul 26;(7):CD003106. doi: 10.1002/14651858.CD003106.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2018 Oct 05;10:CD003106. doi: 10.1002/14651858.CD003106.pub3. PMID: 23888485 Updated.
References
References to studies included in this review
Duvekot 2015 {published data only}
-
- Duvekot J, Bax C, Bloemenkamp K, Dijk P, Van D, Franssen M, et al. Temporizing management versus termination of pregnancy in women with severe preeclampsia at 28‐34 weeks (TOTEM‐Trial). American Journal of Obstetrics and Gynecology 2015;212(1 Suppl 1):S246.
-
- Duvekot JJ, Steegers EAP, Hop WCJ, Franx A, Post JAM, Wassenaer A, et al. TOTEM study (temporise or terminate pregnancy in women with severe preeclampsia at 28‐34 weeks): a study protocol. Pregnancy Hypertension 2011;1 Suppl 1:295. - PubMed
-
- NTR2986. Expectant or active management in women with severe preeclampsia at 28‐34 weeks. Available at trialregister.nl/trialreg/admin/rctview.asp?TC=2986 (first received 11 July 2011).
Mesbah 2003 {published data only}
-
- Mesbah EMM. Severe preterm preeclampsia: aggressive or expectant management?. Medical Journal of Cairo University 2003;71(1):175‐82.
Odendaal 1990 {published data only}
-
- Odendaal HJ, Pattinson RC. Active or conservative treatment of severe preeclampsia ‐ a randomized control trial. Proceedings of 37th Annual Meeting of the Society for Gynecologic Investigation; 1990 March 21‐24; St Louis, USA. 1990:359.
-
- Odendaal HJ, Pattinson RC, Bam R, Grove D, Kotze TJ. Aggressive or expectant management for patients with severe preeclampsia between 28‐34 weeks' gestation: a randomized controlled trial. Obstetrics & Gynecology 1990;76:1070‐5. - PubMed
Sibai 1994 {published data only}
-
- Sibai BM, Mercer BM, Schiff E, Friedman SA. Aggressive versus expectant management of severe preeclampsia at 28 to 32 weeks' gestation: a randomized controlled trial. American Journal of Obstetrics and Gynecology 1994;171:818‐22. - PubMed
Thornton 2004 (GRIT) {published data only}
-
- GRIT Study Group. A randomised trial of timed delivery for the compromised preterm fetus: short term outcomes and Bayesian interpretation. BJOG: An International Journal of Obstetrics and Gynaecology 2003;110:27‐32. - PubMed
-
- Hornbuckle J, Thornton JG, Vail A, GRIT SG. The Growth Restriction Intervention Trial (GRIT): interim progress report. Women's Health ‐ into the new millennium. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 October 3‐6; Cape Town South Africa. RCOG, 1999:76.
-
- Schneider KTM, Brocker J, Thornton J. A randomised intervention of timed delivery for the compromised preterm fetus [Randomisierte Intervention zur Optimierung des Entbindungszeitpunktes bei kompromittierten IUGR‐Feten (Euro‐GRIT)]. Geburtshilfe und Frauenheilkunde 2000;60 Suppl 1:S72.
-
- Thornton JG, Hornbuckle J, Vail A, Spiegelhalter DJ, Levene M, GRIT study group. Infant wellbeing at 2 years of age in the Growth Restriction Intervention Trial (GRIT): multicentred randomised controlled trial. Lancet 2004;364:513‐20. - PubMed
-
- Walker DM, Marlow N, Upstone L, Gross H, Hornbuckle J, Vail A, et al. The Growth Restriction Intervention Trial: long‐term outcomes in a randomized trial of timing of delivery in fetal growth restriction. American Journal of Obstetrics and Gynecology 2011;204(1):34.e1‐9. - PubMed
Vigil‐De Gracia 2013 {published data only}
-
- NCT01164852. Expectant management of severe preeclampsia at 28 to 33 week`s gestation: a randomized controlled trial. Available at clinicaltrials.gov/ct2/show/NCT01164852 (first received 19 July 2010).
-
- Vigil‐De Gracia P, Reyes Tejada O, Calle Minaca A, Tellez G, Chon VY, Herrarte E, et al. Expectant management of severe preeclampsia remote from term: the MEXPRE Latin Study, a randomized, multicenter clinical trial. American Journal of Obstetrics and Gynecology 2013;209(5):425.e1‐8. - PubMed
References to studies excluded from this review
Gruppo di Studio1998 {published data only}
-
- Gruppo di Studio Ipertensione in Gravidanza. Nifedipine versus expectant management in mild to moderate hypertension in pregnancy. British Journal of Obstetrics and Gynaecology 1998;105:718‐22. - PubMed
Langenveld 2011 {published data only}
-
- Langenveld J, Broekhuijsen K, Baaren GJ, Pampus MG, Kaam AH, Groen H, et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre‐eclampsia between 34 and 37 weeks' gestation (HYPITAT‐II): A multicentre, open‐label randomised controlled trial. BMC Pregnancy and Childbirth 2011;11:50. - PMC - PubMed
Additional references
Brown 2001
-
- Brown MA, Lindheimer MD, Swiet M, Assche A, Moutquin J‐M. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy. Hypertension in Pregnancy 2001;20:ix‐xiv. - PubMed
Crowley 1996
Davey 1988
-
- Davey DA, MacGillivray I. The classification and definition of the hypertensive disorders in pregnancy. American Journal of Obstetrics and Gynecology 1988;158:892‐8. - PubMed
Derham 1989
-
- Derham RJ, Hawkins DF, deVries LS, Aber VR, Elder MG. Outcome of pregnancies complicated by severe hypertension and delivered before 34 weeks: stepwise logistic regression analysis of prognostic factors. British Journal of Obstetrics and Gynaecology 1989;96:1173‐81. - PubMed
Duley 1999a
Duley 1999b
Duley 2005
Duley 2007
Duley 2009
-
- Duley L, Henderson‐Smart DJ, Walker GJA. Interventions for treating pre‐eclampsia and its consequences: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007756] - DOI
Duley 2010
Duley 2013
Gillon 2014
GRADE Handbook
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed prior to 25 September 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Higgins 2011
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hofmeyr 2014
Magee 2009
-
- Magee LA, Yong PJ, Espinosa V, Cote AM, Chen I, Dadelszen P. Expectant management of severe pre‐eclampsia remote from term: a structured systematic review. Hypertension in Pregnancy 2009;28(3):312‐47. - PubMed
Makrides 2014
Morris 2012
-
- Morris RK, Riley RD, Doug M, Deeks JJ, Kilby MD. Diagnostic accuracy of spot urinary protein and albumin to creatinine ratios for detection of significant proteinuria or adverse pregnancy outcome in patients with suspected pre‐eclampsia: systematic review and meta‐analysis. BMJ (Clinical research ed.) 2012;345:e4342. [PUBMED: 22777026] - PMC - PubMed
NICE 2010
-
- National Collaborating Centre for Women’s and Children’s Health. Hypertension in pregnancy: the management of hypertensive disorders during pregnancy (NICE Clinical Guidelines, No. 107). London: RCOG Press, 2010. - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2013
Sibai 1984
-
- Sibai BM, Spinnato JA, Watson DL, Hill GA, Anderson GD. Pregnancy outcome in 303 cases with severe preeclampsia. Obstetrics & Gynecology 1984;64:319‐25. - PubMed
von Dadelszen 2011
-
- Dadelszen P, Payne B, Li J, Ansermino MJ, Broughton FP, Cote A, et al. Prediction of adverse maternal outcomes in pre‐eclampsia: development and validation of the full PIERS model. Lancet 2011;377(9761):219‐27. - PubMed
WHO 2011
-
- World Health Organization. WHO recommendations for prevention and treatment of pre‐eclampsia and eclampsia. www.who.int/reproductivehealth/publications/maternal_perinatal_health/97... 2011:1‐48. - PubMed
References to other published versions of this review
Churchill 2002
Churchill 2013
Duley 1995
-
- Duley L. Aggressive vs expectant management of pre‐eclampsia. In: Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C, editor(s). Pregnancy and Childbirth Module. In: The Cochrane Database of Systematic Reviews (database on disk and CDROM). The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

