Ligand Density and Nanoparticle Clustering Cooperate in the Multivalent Amplification of Epidermal Growth Factor Receptor Activation
- PMID: 30289688
- PMCID: PMC6252274
- DOI: 10.1021/acsnano.8b06141
Ligand Density and Nanoparticle Clustering Cooperate in the Multivalent Amplification of Epidermal Growth Factor Receptor Activation
Abstract
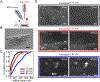
Multivalent presentation of ligands on nanoparticles (NPs) is considered a general strategy for enhancing receptor binding and activation through amplification of ligand-receptor interactions within the footprint of the individual NPs. The spatial clustering of ligand-functionalized NPs represents an additional, less well understood mechanism for increasing local ligand-receptor interactions, especially for receptors that form higher-order assemblies, such as the epidermal growth factor (EGF) receptor (EGFR). To shed light on the interplay between ligand density ( i.e., multivalency) and NP clustering in signal amplification, we apply EGF-functionalized 72 ± 1 nm gold nanoparticles (NP-EGF) with known ligand loading (10-200 EGF/NP) as quantifiable and experimentally tractable units of EGFR activation and characterize the NP-mediated amplification of EGFR phosphorylation as a function of both EGF surface density and NP-EGF clustering for two cancer cell lines (HeLa and MDA-MB-468). The measurements confirm a strong multivalent amplification of EGFR phosphorylation through NP-EGF on the cellular level that results in EGF-loading-dependent maximum EGFR phosphorylation levels. A microscopic analysis of NP-EGF-induced EGFR phosphorylation reveals a heterogeneous spatial distribution of EGFR activation across the cell surface. Clustering of multivalent NP-EGF on sub-diffraction-limited length scales is found to result in a local enhancement of EGFR phosphorylation in signaling "hot spots" from where the signal can spread laterally in an EGF-independent fashion. Increasing EGF loadings of the NP enhances NP-EGF clustering and intensifies EGFR phosphorylation. These observations suggest that NP-EGF clustering and the associated local enhancement of ligand-receptor interactions are intrinsic components of the multivalent amplification of phosphorylation for the heterogeneously distributed EGFR through NP-EGF.
Keywords: cell signaling; epidermal growth factor receptor; gold nanoparticles; multivalency; nanoparticle−cell interactions.
Figures





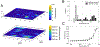
References
-
- Schlessinger J Ligand-Induced, Receptor-Mediated Dimerization and Activation of EGF Receptor. Cell 2002, 110, 669–672. - PubMed
-
- Schlessinger J Cell Signaling by Receptor Tyrosine Kinases. Cell 2000, 103, 211–225. - PubMed
-
- Yarden Y; Schlessinger J Self-Phosphorylation of Epidermal Growth Factor Receptor: Evidence for a Model of Intermolecular Allosteric Activation. Biochemistry 1987, 26, 1434–1442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

