Phase I clinical trial of an intranodally administered mRNA-based therapeutic vaccine against HIV-1 infection
- PMID: 30289805
- PMCID: PMC6221380
- DOI: 10.1097/QAD.0000000000002026
Phase I clinical trial of an intranodally administered mRNA-based therapeutic vaccine against HIV-1 infection
Erratum in
-
Preclinical evaluation of an mRNA HIV vaccine combining rationally selected antigenic sequences and adjuvant signals (HTI-TriMix): Erratum Phase I clinical trial of an intranodally administered mRNA-based therapeutic vaccine against HIV-1 infection: Erratum.AIDS. 2019 Oct 1;33(12):1957. doi: 10.1097/01.aids.0000579184.27339.a8. AIDS. 2019. PMID: 31490219 Free PMC article. No abstract available.
Abstract
Objective: The efficacy of therapeutic vaccines against HIV-1 infection has been modest. New inerts to redirect responses to vulnerable sites are urgently needed to improve these results.
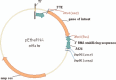
Design: We performed the first-in-human clinical trial with naked mRNA (iHIVARNA) combining a dendritic cell activation strategy (TriMix:CD40L+CD70+caTLR4 RNA) with a novel HIV immunogen sequences (HTI immunogen).
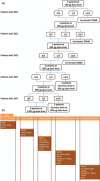
Methods: A dose escalation, phase I clinical trial was performed in 21 chronic HIV-1-infected patients under ART who received three intranodal doses of mRNA (weeks 0, 2 and 4) as follow: TriMix-100 g, TriMix-300 g, TriMix-300 g with HTI-300 g, TriMix-300 g with HTI-600 g, TriMix-300 g with HTI-900 g. Primary end-point was safety and secondary-exploratory end-points were immunogenicity, changes in viral reservoir and transcriptome.
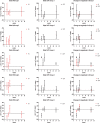
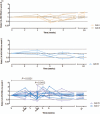
Results: Overall, the vaccine was secure and well tolerated. There were 31 grade 1/2 and 1 grade 3 adverse events, mostly unrelated to the vaccination. Patients who received the highest dose showed a moderate increase in T-cell responses spanning HTI sequence at week 8. In addition, the proportion of responders receiving any dose of HTI increased from 31% at w0 to 80% postvaccination. The intervention had no impact on caHIV-DNA levels, however, caHIV-RNA expression and usVL were transiently increased at weeks 5 and 6 in the highest dose of iHIVARNA, and these changes were positively correlated with HIV-1-specific-induced immune responses.
Conclusion: This phase I dose-escalating trial showed that iHIVARNA administration was safe and well tolerated, induced moderate HIV-specific T-cell responses and transiently increased different viral replication readouts. These data support further exploration of iHIVARNA in a phase II study. CLINICALTRIALS.
Gov identifier: NCT02413645.
Figures



References
-
- Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998; 338:853–860. - PubMed
-
- Campbell C, Ambrosioni J, Miro JM, Esteve A, Casabona J, Navarro G, et al. The continuum of HIV care in Catalonia. AIDS Care 2015; 27:1449–1454. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

