Impact of current smoking on 2-year clinical outcomes between durable-polymer-coated stents and biodegradable-polymer-coated stents in acute myocardial infarction after successful percutaneous coronary intervention: Data from the KAMIR
- PMID: 30289945
- PMCID: PMC6173404
- DOI: 10.1371/journal.pone.0205046
Impact of current smoking on 2-year clinical outcomes between durable-polymer-coated stents and biodegradable-polymer-coated stents in acute myocardial infarction after successful percutaneous coronary intervention: Data from the KAMIR
Abstract
Objective: Data concerning the effect of current smoking on solely new-generation drug-eluting stents (DES) are limited. We investigated the impact of current smoking on 2-year clinical outcomes between durable-polymer (DP)-coated DES (zotarolimus-eluting [ZES] and everolimus eluting [EES]) and biodegradable-polymer (BP)-coated biolimus-eluting stent (BES) in acute myocardial infarction (AMI) patients after successful percutaneous coronary intervention (PCI).
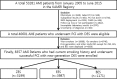
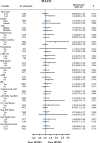
Methods: Finally, a total of 8357 AMI patients with current smoking underwent successful PCI with new-generation DES (ZES, EES, and BES) were enrolled and divided into three groups as ZES (n = 3199), EES (n = 3987), and BES group (n = 1171). The primary endpoint was the occurrence of major adverse cardiac events (MACE) defined as all-cause death (cardiac death [CD] or non-cardiac death), recurrent AMI (re-MI), any revascularization (target lesion revascularization [TLR], target vessel revascularization [TVR], and non-TVR). The secondary endpoint was the incidence of definite or probable stent thrombosis (ST).
Results: The 2-year adjusted hazard ratio (HR) of MACE for ZES vs. EES (1.055; 95% confidence interval [CI], 0.843-1.321; p = 0.638), ZES vs. BES (HR, 0.885; 95% CI, 0.626-1.251; p = 0.488), EES vs. BES (HR, 0.889; 95% CI, 0.633-1.250; p = 0.499), and ZES/EES vs. BES (HR, 0.891; 95% CI, 0.648-1.126; p = 0.480) were similar. The occurrence of ST after adjustment were also comparable. In addition, the 2-year adjusted HR for all-cause death, CD, re-MI, TLR, TVR, and non-TVR were not different.
Conclusions: In this study, DP-DES and BP-DES showed comparable safety and efficacy during 2-year follow-up periods. Therefore, DP-DES or BP-DES are equally acceptable in AMI patients with current smoking undergoing PCI.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Burns DM, Epidemiology of smoking-induced cardiovascular disease. Prog Cardiovasc Dis. 2003;46(1):11–29. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

