Real Time Normalization of Fast Photochemical Oxidation of Proteins Experiments by Inline Adenine Radical Dosimetry
- PMID: 30290117
- PMCID: PMC7811273
- DOI: 10.1021/acs.analchem.8b02787
Real Time Normalization of Fast Photochemical Oxidation of Proteins Experiments by Inline Adenine Radical Dosimetry
Abstract
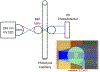
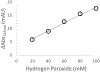
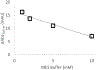
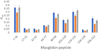
Hydroxyl radical protein footprinting (HRPF) is a powerful method for measuring protein topography, allowing researchers to monitor events that alter the solvent accessible surface of a protein (e.g., ligand binding, aggregation, conformational changes, etc.) by measuring changes in the apparent rate of reaction of portions of the protein to hydroxyl radicals diffusing in solution. Fast Photochemical Oxidation of Proteins (FPOP) offers an ultrafast benchtop method for radical generation for HRPF, photolyzing hydrogen peroxide using a UV laser to generate high concentrations of hydroxyl radicals that are consumed on roughly a microsecond time scale. The broad reactivity of hydroxyl radicals means that almost anything added to the solution (e.g., ligands, buffers, excipients, etc.) will scavenge hydroxyl radicals, altering their half-life and changing the effective radical concentration experienced by the protein. Similarly, minute changes in peroxide concentration, laser fluence, and buffer composition can alter the effective radical concentration, making reproduction of data challenging. Here, we present a simple method for radical dosimetry that can be carried out as part of the FPOP workflow, allowing for measurement of effective radical concentration in real time. Additionally, by modulating the amount of radical generated, we demonstrate that effective hydroxyl radical yields in FPOP HRPF experiments carried out in buffers with widely differing levels of hydroxyl radical scavenging capacity can be compensated on the fly, yielding statistically indistinguishable results for the same conformer. This method represents a major step in transforming FPOP into a robust and reproducible technology capable of probing protein structure in a wide variety of contexts.
Conflict of interest statement
Conflict of Interest Disclosure
J.S.S. and S.R.W. disclose a significant financial interest in GenNext Technologies, Inc., an early-stage company seeking to commercialize technologies for protein higher order structure analysis. This manuscript and all data were reviewed by S.K.M., who has no financial conflict of interest, in accordance with University of Mississippi FCOI management practices.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

