Potent in vivo lung cancer Wnt signaling inhibition via cyclodextrin-LGK974 inclusion complexes
- PMID: 30290244
- PMCID: PMC6441337
- DOI: 10.1016/j.jconrel.2018.09.025
Potent in vivo lung cancer Wnt signaling inhibition via cyclodextrin-LGK974 inclusion complexes
Abstract
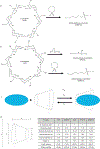
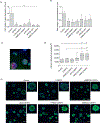
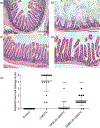
Activation of the Wnt signaling pathway promotes lung cancer progression and contributes to poor patient prognosis. The porcupine inhibitor LGK974, a novel orally bioavailable cancer therapeutic in Phase I clinical trials, induces potent Wnt signaling inhibition and leads to suppressed growth and progression of multiple types of cancers. The clinical use of LGK974, however, is limited in part due to its low solubility and high toxicity in tissues that rely on Wnt signaling for normal homeostasis. Here, we report the use of host-guest chemistry to enhance the solubility and bioavailability of LGK974 in mice through complexation with cyclodextrins (CD). We assessed the effects of these complexes to inhibit Wnt signaling in lung adenocarcinomas that are typically driven by overactive Wnt signaling. 2D 1H NMR confirmed host-guest complexation of CDs with LGK974. CD:LGK974 complexes significantly decreased the expression of Wnt target genes in lung cancer organoids and in lung cancer allografts in mice. Further, CD:LGK974 complexes increased the bioavailability upon oral administration in mice compared to free LGK974. In a mouse lung cancer allograft model, CD:LGK974 complexes induced potent Wnt signaling inhibition with reduced intestinal toxicity compared to treatment with free drug. Collectively, the development of these complexes enables safer and repeated oral or parenteral administration of Wnt signaling inhibitors, which hold promise for the treatment of multiple types of malignancies.
Copyright © 2018. Published by Elsevier B.V.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

