Everolimus-induced pulmonary toxicity: Findings on 18F-FDG PET/CT imaging
- PMID: 30290608
- PMCID: PMC6200531
- DOI: 10.1097/MD.0000000000012518
Everolimus-induced pulmonary toxicity: Findings on 18F-FDG PET/CT imaging
Abstract
The everolimus-exemestane combination is indicated in advanced breast cancer treatment and usually well tolerated. The objective of the study was to determine the frequency of everolimus lung side effects and investigate their imaging characteristics on positron emission tomography with 18F-fluoro-deoxy-glucose combined with computerized tomography (F-FDG PET/CT).Our single-center retrospective descriptive study systematically included all patients with metastatic breast cancer treated by this combination (n = 29 representing 57 F-FDG PET/CT). Number of segments involved was quantified. Maximum standardized uptake value (SUVmax), average standardized uptake value (SUVmean), metabolic target volume (MTV), and total lesion glycolysis (TLG) were measured. Severe pneumopathy was studied by subgroup analysis.Pleuroparenchymal anomalies rate detected on F-FDG PET/CT was 62%. Alveolar-interstitial lesions were mainly observed (89%) and affected 2.8 segments (0.5-11.5) with a median of 2 segments. S7 and S10 were the most involved segments with SUVmax 3.9 (1.3-8.8) and SUVmean 2.2 (0.7-4.9). Statistically significant difference (P = .02) was found with number of segment involved to characterize severe pneumopathy (average of 6.3 segments [2.5-11.5] vs 1.9 segments [0.5-8] for interstitial lung disease) but not with SUVmax, SUVmean, MTV, TLG (P = .14, 0.22, 0.22, and 0.17, respectively).The F-FDG PET/CT could highlight pulmonary everolimus side effects, with a typical imaging pattern: alveolar-interstitial opacities associated with moderate uptake, more or less extensive, mainly affecting the lower lobes. Rarely, a pseudotumoral aspect may be detected, corresponding to a pitfall. MTV or TLG showed a tendency to differentiate severe pneumopathy vs interstitial lung disease but no statistically significant differences was observed contrarily to the number of segments involved. Further studies are necessary to determine if the F-FDG PET/CT could early predict adverse effects of mTOR inhibitors.
Conflict of interest statement
The authors have no funding and conflicts of interest to disclose.
Figures
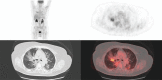
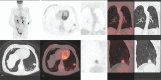
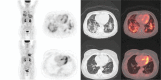
References
-
- Ortolani S, Ciccarese C, Cingarlini S, et al. Suppression of mTOR pathway in solid tumors: lessons learned from clinical experience in renal cell carcinoma and neuroendocrine tumors and new perspectives. Future Oncol 2015;11:1809–28. - PubMed
-
- Li N, Hao Y, Xie J, et al. Effectiveness of everolimus versus endocrine monotherapy or chemotherapy among HR+/HER2- mBC patients with multiple metastatic sites. Clin Ther 2016;38:905–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

