Genomic Differences Between "Primary" and "Secondary" Muscle-invasive Bladder Cancer as a Basis for Disparate Outcomes to Cisplatin-based Neoadjuvant Chemotherapy
- PMID: 30290956
- PMCID: PMC6339572
- DOI: 10.1016/j.eururo.2018.09.002
Genomic Differences Between "Primary" and "Secondary" Muscle-invasive Bladder Cancer as a Basis for Disparate Outcomes to Cisplatin-based Neoadjuvant Chemotherapy
Abstract
Background: Cisplatin-based neoadjuvant chemotherapy (NAC) followed by radical cystectomy (RC) is the standard of care for patients with muscle-invasive bladder cancer (MIBC). It is unknown whether this treatment strategy is appropriate for patients who progress to MIBC after treatment for prior noninvasive disease (secondary MIBC).
Objective: To determine whether clinical and genomic differences exist between primary and secondary MIBC treated with NAC and RC.
Design, setting, and participants: Clinicopathologic outcomes were compared between 245 patients with clinical T2-4aN0M0-stage primary MIBC and 43 with secondary MIBC treated with NAC and RC at Memorial Sloan Kettering Cancer Center (MSKCC) from 2001 to 2015. Genomic differences were assessed in a retrospective cohort of 385 prechemotherapy specimens sequenced by whole-exome or targeted exon capture by the Cancer Genome Atlas or at MSKCC. Findings were confirmed in an independent validation cohort of 94 MIBC patients undergoing prospective targeted exon sequencing at MSKCC.
Outcome measurements and statistical analysis: Pathologic response rates, recurrence-free survival (RFS), bladder cancer-specific survival (CSS), and overall survival (OS) were measured. Differences in somatic genomic alteration rates were compared using Fisher's exact test and the Benjamini-Hochberg false discovery rate method.
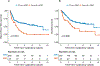
Results and limitations: Patients with secondary MIBC had lower pathologic response rates following NAC than those with primary MIBC (univariable: 26% vs 45%, multivariable: odds ratio=0.4 [95% confidence interval=0.18-0.84] p=0.02) and significantly worse RFS, CSS, and OS. Patients with secondary MIBC treated with NAC had worse CSS compared with cystectomy alone (p=0.002). In a separate genomic analysis, we detected significantly more likely deleterious somatic ERCC2 missense mutations in primary MIBC tumors in both the discovery (10.9% [36/330] vs 1.8% [1/55], p=0.04) and the validation (15.7% [12/70] vs 0% [0/24], p=0.03) cohort.
Conclusions: Patients with secondary MIBC treated with NAC had worse clinical outcomes than similarly treated patients with primary MIBC. ERCC2 mutations predicted to result in increased cisplatin sensitivity were enriched in primary versus secondary MIBC. Prospective validation is still needed, but given the lack of clinical benefit with cisplatin-based NAC in patients with secondary MIBC, upfront RC or enrollment in clinical trials should be considered.
Patient summary: A retrospective cohort study of patients with "primary" and "secondary" muscle-invasive bladder cancer (MIBC) treated with chemotherapy before surgical removal of the bladder identified lower response rates and shorter survival in patients with secondary MIBC. Tumor genetic sequencing of separate discovery and validation cohorts revealed that chemotherapy-sensitizing DNA damage repair gene mutations occur predominantly in primary MIBC tumors and may underlie the greater sensitivity of primary MIBC to chemotherapy. Prospective validation is still needed, but patients with secondary MIBC may derive greater benefit from upfront surgery or enrollment in clinical trials rather than from standard chemotherapy.
Keywords: Bladder cancer; ERCC2; Muscle-invasive bladder cancer; Neoadjuvant chemotherapy; Non–muscle-invasive bladder cancer; Radical cystectomy.
Copyright © 2018 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Pietzak: Scientific Advisory Board: Merck. Berger: Consultant: Roche; Research Support: Illumina. Funt: Research Support: Genentech, AstraZeneca; Stock ownership: Kite Pharma, Urogen Pharma, Allogene Therapeutics, Kronos Bio. Bajorin: Honoraria from the speaker’s bureau of Merck; Consultant/advisory board: Merck, Pfizer, Bristol-Myers Squibb, Urogen, Genentech, Eli Lilly. Rosenberg: Stock and Other Ownership Interests: Merck, Illumina; Honoraria: UpToDate, Bristol-Myers Squibb, AstraZeneca, Medscape, Vindico, Peerview, Chugai Pharma; Consulting or Advisory Role: Lilly, Merck, Agensys, Roche/Genentech, Sanofi, AstraZeneca/MedImmune, Bristol-Myers Squibb, EMD Serono, Seattle Genetic, Bayer, Inovio Pharmaceuticals, BioClin Therapeutics, QED Therapeutic, Adicet Bio, Sensei Biotherapeutics; Patents, Royalties, Other Intellectual Property: Predictor of platinum sensitivity; Research Funding: Genentech, Oncogenex, Agensys, Mirati Therapeutics, Novartis, Viralytics, Genentech/Roche, Incyte, Seattle Genetics, Bayer; Travel, Accommodations, Expenses: Genentech/Roche, Bristol-Myers Squibb. Bochner: Chair of data safety monitoring committee: Genentech; Course Instructor: Olympus. Al-Ahmadie: Consultant: Bristol-Myers-Squibb, AstraZeneca, EMD Serono. Solit: Consultant for Pfizer, Loxo Oncology and Illumina. Iyer: Consultant: Bayer
Figures

Comment in
-
ERCC2 Mutation: The Marker for Chemosensitivity in Primary and Secondary Muscle-invasive Bladder Cancers.Eur Urol. 2019 Feb;75(2):240-241. doi: 10.1016/j.eururo.2018.09.043. Epub 2018 Oct 23. Eur Urol. 2019. PMID: 30366635 No abstract available.
-
Re: Genomic Differences Between "Primary" and "Secondary" Muscle-invasive Bladder Cancer as a Basis for Disparate Outcomes to Cisplatin-based Neoadjuvant Chemotherapy.Eur Urol. 2019 Apr;75(4):694. doi: 10.1016/j.eururo.2018.12.019. Epub 2019 Jan 17. Eur Urol. 2019. PMID: 30661731 No abstract available.
-
Re: Genomic Differences between "Primary" and "Secondary" Muscle-Invasive Bladder Cancer as a Basis for Disparate Outcomes to Cisplatin-Based Neoadjuvant Chemotherapy.J Urol. 2019 Jul;202(1):30. doi: 10.1097/01.JU.0000557728.16853.8f. Epub 2019 Jun 7. J Urol. 2019. PMID: 30925134 No abstract available.
-
Biomarkers for platinum sensitivity in bladder cancer: are we there yet?Transl Androl Urol. 2019 Jul;8(Suppl 3):S236-S239. doi: 10.21037/tau.2019.01.10. Transl Androl Urol. 2019. PMID: 31392132 Free PMC article. No abstract available.
-
Neoadjuvant cisplatin-based chemotherapy in "primary" and "secondary" muscle-invasive bladder cancer-is it a surrogate for molecular subtypes?Transl Cancer Res. 2019 Mar;8(Suppl 2):S176-S179. doi: 10.21037/tcr.2019.01.05. Transl Cancer Res. 2019. PMID: 35117093 Free PMC article. No abstract available.
References
-
- Milowsky MI, Rumble RB, Booth CM, et al. Guideline on muscle-invasive and metastatic bladder cancer (European Association of Urology Guideline): American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol 2016;34:1945–52. - PubMed
-
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859–66. - PubMed
-
- International Collaboration of Trialists, Medical Research Council Advanced Bladder Cancer Working Party, European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol 2011;29:2171–7. - PMC - PubMed
-
- Manoharan M, Katkoori D, Kishore TA, Kava B, Singal R, Soloway MS. Outcome after radical cystectomy in patients with clinical T2 bladder cancer in whom neoadjuvant chemotherapy has failed. BJU Int 2009;104:1646–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

