Mechanistic basis for the evolution of chalcone synthase catalytic cysteine reactivity in land plants
- PMID: 30291143
- PMCID: PMC6290136
- DOI: 10.1074/jbc.RA118.005695
Mechanistic basis for the evolution of chalcone synthase catalytic cysteine reactivity in land plants
Abstract
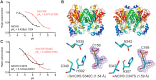
Flavonoids are important polyphenolic natural products, ubiquitous in land plants, that play diverse functions in plants' survival in their ecological niches, including UV protection, pigmentation for attracting pollinators, symbiotic nitrogen fixation, and defense against herbivores. Chalcone synthase (CHS) catalyzes the first committed step in plant flavonoid biosynthesis and is highly conserved in all land plants. In several previously reported crystal structures of CHSs from flowering plants, the catalytic cysteine is oxidized to sulfinic acid, indicating enhanced nucleophilicity in this residue associated with its increased susceptibility to oxidation. In this study, we report a set of new crystal structures of CHSs representing all five major lineages of land plants (bryophytes, lycophytes, monilophytes, gymnosperms, and angiosperms), spanning 500 million years of evolution. We reveal that the structures of CHS from a lycophyte and a moss species preserve the catalytic cysteine in a reduced state, in contrast to the cysteine sulfinic acid seen in all euphyllophyte CHS structures. In vivo complementation, in vitro biochemical and mutagenesis analyses, and molecular dynamics simulations identified a set of residues that differ between basal-plant and euphyllophyte CHSs and modulate catalytic cysteine reactivity. We propose that the CHS active-site environment has evolved in euphyllophytes to further enhance the nucleophilicity of the catalytic cysteine since the divergence of euphyllophytes from other vascular plant lineages 400 million years ago. These changes in CHS could have contributed to the diversification of flavonoid biosynthesis in euphyllophytes, which in turn contributed to their dominance in terrestrial ecosystems.
Keywords: chalcone synthase; cysteine; enzyme catalysis; enzyme mechanism; flavonoid; pKa; plant evolution; polyketide; polyketide synthase; redox regulation.
© 2018 Liou et al.
Conflict of interest statement
J. K. W. is a co-founder, a member of the Scientific Advisory Board, and a shareholder of DoubleRainbow Biosciences, which develops biotechnologies related to natural products
Figures


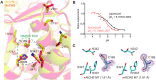


References
-
- Rausher M. D. (2006) in The Science of Flavonoids (Grotewold E., ed) pp. 175–211, Springer, New York
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

