Function and Dysfunction of TMC Channels in Inner Ear Hair Cells
- PMID: 30291150
- PMCID: PMC6450785
- DOI: 10.1101/cshperspect.a033506
Function and Dysfunction of TMC Channels in Inner Ear Hair Cells
Abstract
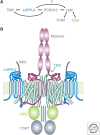
The TMC1 channel was identified as a protein essential for hearing in mouse and human, and recognized as one of a family of eight such proteins in mammals. The TMC family is part of a superfamily of seven branches, which includes the TMEM16s. Vertebrate hair cells express both TMC1 and TMC2. They are located at the tips of stereocilia and are required for hair cell mechanotransduction. TMC1 assembles as a dimer and its similarity to the TMEM16s has enabled a predicted tertiary structure with an ion conduction pore in each subunit of the dimer. Cysteine mutagenesis of the pore supports the role of TMC1 and TMC2 as the core channel proteins of a larger mechanotransduction complex that includes PCDH15 and LHFPL5, and perhaps TMIE, CIB2 and others.
Copyright © 2019 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

References
-
- Alagramam KN, Murcia CL, Kwon HY, Pawlowski KS, Wright CG, Woychik RP. 2001a. The mouse Ames waltzer hearing-loss mutant is caused by mutation of Pcdh15, a novel protocadherin gene. Nat Genet 27: 99–102. - PubMed
-
- Alagramam KN, Yuan H, Kuehn MH, Murcia CL, Wayne S, Srisailpathy CR, Lowry RB, Knaus R, Van Laer L, Bernier FP, et al. 2001b. Mutations in the novel protocadherin PCDH15 cause Usher syndrome type 1F. Hum Mol Genet 10: 1709–1718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
