Heated tobacco product regulation under US law and the FCTC
- PMID: 30291201
- PMCID: PMC6204223
- DOI: 10.1136/tobaccocontrol-2018-054560
Heated tobacco product regulation under US law and the FCTC
Abstract
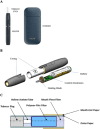
Tobacco companies are marketing new 'heated tobacco products' (HTPs) composed of battery-powered holders, chargers and tobacco plugs or sticks. The non-tobacco HTP components have escaped effective regulation under many countries' tobacco control laws because they are packaged and sold separately from the tobacco-containing components. In the USA, HTPs cannot be marketed unless the Food and Drug Administration determines that allowing their sale would be 'appropriate for the protection of the public health'. Philip Morris International (PMI) is seeking permission to market its IQOS HTP in the USA with 'modified risk tobacco product' (MRTP) claims that it reduces exposure to harmful substances and is less harmful than other tobacco products. However, PMI has not submitted adequate scientific evidence required by US law to demonstrate that the product is significantly less harmful to users than other tobacco products, that its labelling would not mislead consumers, or that its marketing-with or without MRTP claims-would benefit the health of the population as a whole. Parties to the WHO Framework Convention on Tobacco Control (FCTC) must take measures to reduce tobacco use and nicotine addiction, and prevent false or misleading tobacco product labelling, advertising and promotions; the introduction of new HTPs must be assessed according to these goals. All components of HTPs should be regulated at least as stringently as existing tobacco products, including restrictions on labelling, advertising, promotion and sponsorship, sales to minors, price and taxation policies and smokefree measures. There is nothing in US law or the FCTC that prevents authorities from prohibiting HTPs.
Keywords: advertising and promotion; non-cigarette tobacco products; packaging and labelling; public policy.
© Author(s) (or their employer(s)) 2018. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Harlay J, 2016. What you need to know about Heat-not-Burn (HNB) cigarettes https://www.vapingpost.com/2016/11/09/what-you-need-to-know-about-heat-n... (accessed 14 Feb 2018).
-
- PMI. Science and innovation: our findings to Date. https://www.pmi.com/science-and-innovation/our-findings-to-date (accessed 18 Feb 2018).
-
- U.S. Food and Drug Administration. Family Smoking Prevention and Tobacco Control Act, Pub. L. 111-31, 21 U.S.C. 387 et seq. 2009.
-
- World Health Organization, 2005. WHO framework convention on tobacco control http://www.who.int/fctc/en/ (accessed 1 Feb 2018).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources