Instantaneous Midbrain Control of Saccade Velocity
- PMID: 30291204
- PMCID: PMC6246878
- DOI: 10.1523/JNEUROSCI.0962-18.2018
Instantaneous Midbrain Control of Saccade Velocity
Abstract
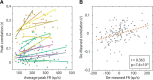
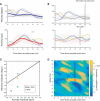
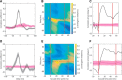
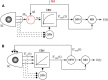
The ability to interact with our environment requires the brain to transform spatially represented sensory signals into temporally encoded motor commands for appropriate control of the relevant effectors. For visually guided eye movements, or saccades, the superior colliculus (SC) is assumed to be the final stage of spatial representation, and instantaneous control of the movement is achieved through a rate code representation in the lower brain stem. We investigated whether SC activity in nonhuman primates (Macaca mulatta, 2 male and 1 female) also uses a dynamic rate code, in addition to the spatial representation. Noting that the kinematics of amplitude-matched movements exhibit trial-to-trial variability, we regressed instantaneous SC activity with instantaneous eye velocity and found a robust correlation throughout saccade duration. Peak correlation was tightly linked to time of peak velocity, the optimal efferent delay between SC activity and eye velocity was constant at ∼12 ms both at onset and during the saccade, and SC neurons with higher firing rates exhibited stronger correlations. Moreover, the strong correlative relationship and constant efferent delay observation were preserved when eye movement profiles were substantially altered by a blink-induced perturbation. These results indicate that the rate code of individual SC neurons can control instantaneous eye velocity and argue against a serial process of spatial-to-temporal transformation. They also motivated us to consider a new framework of saccade control that does not incorporate traditionally accepted elements, such as the comparator and resettable integrator, whose neural correlates have remained elusive.SIGNIFICANCE STATEMENT All movements exhibit time-varying features that are under instantaneous control of the innervating neural command. At what stage in the brain is dynamical control present? It is well known that, in the skeletomotor system, neurons in the motor cortex use dynamical control. In the oculomotor system, in contrast, instantaneous velocity control of saccadic eye movements is not thought to be enforced until the lower brainstem. Using correlations between residual signals across trials, we show that instantaneous control of saccade velocity is present earlier in the visuo-oculomotor neuraxis, at the level of superior colliculus. The results require us to consider alternate frameworks of the neural control of saccades.
Keywords: efference copy; local feedback model; motor execution; movement variability; neural integrator; oculomotor.
Copyright © 2018 the authors 0270-6474/18/3810156-12$15.00/0.
Figures

Comment in
-
A dynamic, imperturbable link between midbrain activity and saccade velocity.J Neurophysiol. 2020 Feb 1;123(2):451-453. doi: 10.1152/jn.00328.2019. Epub 2019 Oct 2. J Neurophysiol. 2020. PMID: 31577527 Free PMC article.
Similar articles
-
Evidence that the superior colliculus participates in the feedback control of saccadic eye movements.J Neurophysiol. 2002 Feb;87(2):679-95. doi: 10.1152/jn.00886.2000. J Neurophysiol. 2002. PMID: 11826037
-
Blink-perturbed saccades in monkey. II. Superior colliculus activity.J Neurophysiol. 2000 Jun;83(6):3430-52. doi: 10.1152/jn.2000.83.6.3430. J Neurophysiol. 2000. PMID: 10848560
-
Optimal control of saccades by spatial-temporal activity patterns in the monkey superior colliculus.PLoS Comput Biol. 2012;8(5):e1002508. doi: 10.1371/journal.pcbi.1002508. Epub 2012 May 17. PLoS Comput Biol. 2012. PMID: 22615548 Free PMC article.
-
The deep layers of the superior colliculus.Rev Oculomot Res. 1989;3:213-55. Rev Oculomot Res. 1989. PMID: 2486324 Review.
-
Causal Role of Neural Signals Transmitted From the Frontal Eye Field to the Superior Colliculus in Saccade Generation.Front Neural Circuits. 2018 Aug 28;12:69. doi: 10.3389/fncir.2018.00069. eCollection 2018. Front Neural Circuits. 2018. PMID: 30210307 Free PMC article. Review.
Cited by
-
Sensory tuning in neuronal movement commands.Proc Natl Acad Sci U S A. 2023 Sep 19;120(38):e2305759120. doi: 10.1073/pnas.2305759120. Epub 2023 Sep 11. Proc Natl Acad Sci U S A. 2023. PMID: 37695898 Free PMC article.
-
Impairment but not abolishment of express saccades after unilateral or bilateral inactivation of the frontal eye fields.J Neurophysiol. 2020 May 1;123(5):1907-1919. doi: 10.1152/jn.00191.2019. Epub 2020 Apr 8. J Neurophysiol. 2020. PMID: 32267202 Free PMC article.
-
Multiple groups of neurons in the superior colliculus convert value signals into saccadic vigor.bioRxiv [Preprint]. 2025 Jun 25:2025.06.24.661386. doi: 10.1101/2025.06.24.661386. bioRxiv. 2025. PMID: 40667199 Free PMC article. Preprint.
-
Frontal eye field inactivation alters the readout of superior colliculus activity for saccade generation in a task-dependent manner.J Comput Neurosci. 2021 Aug;49(3):229-249. doi: 10.1007/s10827-020-00760-7. Epub 2020 Nov 8. J Comput Neurosci. 2021. PMID: 33161507
-
A dynamic, imperturbable link between midbrain activity and saccade velocity.J Neurophysiol. 2020 Feb 1;123(2):451-453. doi: 10.1152/jn.00328.2019. Epub 2019 Oct 2. J Neurophysiol. 2020. PMID: 31577527 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
