Temporal Contingencies Determine Whether Adaptation Strengthens or Weakens Normalization
- PMID: 30291205
- PMCID: PMC6246879
- DOI: 10.1523/JNEUROSCI.1131-18.2018
Temporal Contingencies Determine Whether Adaptation Strengthens or Weakens Normalization
Abstract
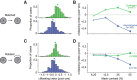
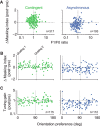
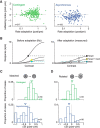
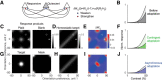
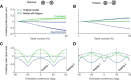
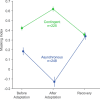
A fundamental and nearly ubiquitous feature of sensory encoding is that neuronal responses are strongly influenced by recent experience, or adaptation. Theoretical and computational studies have proposed that many adaptation effects may result in part from changes in the strength of normalization signals. Normalization is a "canonical" computation in which a neuron's response is modulated (normalized) by the pooled activity of other neurons. Here, we test whether adaptation can alter the strength of cross-orientation suppression, or masking, a paradigmatic form of normalization evident in primary visual cortex (V1). We made extracellular recordings of V1 neurons in anesthetized male macaques and measured responses to plaid stimuli composed of two overlapping, orthogonal gratings before and after prolonged exposure to two distinct adapters. The first adapter was a plaid consisting of orthogonal gratings and led to stronger masking. The second adapter presented the same orthogonal gratings in an interleaved manner and led to weaker masking. The strength of adaptation's effects on masking depended on the orientation of the test stimuli relative to the orientation of the adapters, but was independent of neuronal orientation preference. Changes in masking could not be explained by altered neuronal responsivity. Our results suggest that normalization signals can be strengthened or weakened by adaptation depending on the temporal contingencies of the adapting stimuli. Our findings reveal an interplay between two widespread computations in cortical circuits, adaptation and normalization, that enables flexible adjustments to the structure of the environment, including the temporal relationships among sensory stimuli.SIGNIFICANCE STATEMENT Two fundamental features of sensory responses are that they are influenced by adaptation and that they are modulated by the activity of other nearby neurons via normalization. Our findings reveal a strong interaction between these two aspects of cortical computation. Specifically, we show that cross-orientation masking, a form of normalization, can be strengthened or weakened by adaptation depending on the temporal contingencies between sensory inputs. Our findings support theoretical proposals that some adaptation effects may involve altered normalization and offer a network-based explanation for how cortex adjusts to current sensory demands.
Keywords: V1; adaptation; cross-orientation suppression; macaque.
Copyright © 2018 the authors 0270-6474/18/3810129-14$15.00/0.
Figures


References
-
- Barlow HB. (1990) A theory about the functional role and synaptic mechanism of visual aftereffects. In: Vision: coding and efficiency (Blakemore CB, ed), pp 363–375. New York: Cambridge University.
-
- Barlow HB, Földiák P (1989) Adaptation and decorrelation in the cortex. In: The computing neuron (Durbin R, Miall C, Mitchison G, eds), pp 54–72. New York: Addison-Wesley.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
