AXL Is a Putative Tumor Suppressor and Dormancy Regulator in Prostate Cancer
- PMID: 30291220
- PMCID: PMC6359976
- DOI: 10.1158/1541-7786.MCR-18-0718
AXL Is a Putative Tumor Suppressor and Dormancy Regulator in Prostate Cancer
Abstract
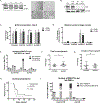
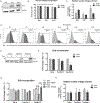
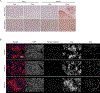
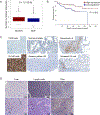
Prostate cancer bone metastasis remains lethal and incurable, and often arises years after elimination of the primary tumor. It is unclear what underlies the decades-long clinical latency before recurrence, but evidence points to the existence of dormant residual tumor cells that disseminated before the primary tumor was eliminated. To design therapies to prevent progression of disseminated tumor cells (DTC) into lethal metastases, it is crucial to understand the mechanism(s) underlying this dormancy. The current study functionally validated our previous observation that implicated the GAS6/AXL axis in mediating DTC dormancy in the bone marrow. AXL-null and AXL-overexpressing prostate cancer cell lines were generated to determine if AXL was necessary and/or sufficient for dormancy. Characterization of these cells in vitro and using in vivo mouse models of DTC growth demonstrated that AXL was indeed sufficient to induce dormancy, but was unable to maintain it long-term and was not absolutely required for a dormancy period. Clinically, AXL expression correlated with longer survival in prostate cancer patients, and AXL was not expressed by cancer cells in primary or metastatic tissue. These data point to a tumor-suppressive role for AXL in prostate cancer, and future work is required to determine if AXL is expressed on human bone marrow DTCs. IMPLICATIONS: The ability of AXL to initiate but not maintain dormancy, coupled with its dispensability, suggests that targeting AXL alone will not prevent lethal metastatic outgrowth, and likely a cooperative network of factors exists to mediate long-term cellular dormancy.
©2018 American Association for Cancer Research.
Conflict of interest statement
Disclosure of potential conflicts of interest
The authors declare no potential conflicts of interest.
Figures



Similar articles
-
Hypoxia stabilizes GAS6/Axl signaling in metastatic prostate cancer.Mol Cancer Res. 2012 Jun;10(6):703-12. doi: 10.1158/1541-7786.MCR-11-0569. Epub 2012 Apr 19. Mol Cancer Res. 2012. PMID: 22516347 Free PMC article.
-
GAS6 receptor status is associated with dormancy and bone metastatic tumor formation.PLoS One. 2013 Apr 24;8(4):e61873. doi: 10.1371/journal.pone.0061873. Print 2013. PLoS One. 2013. PMID: 23637920 Free PMC article.
-
Mer Tyrosine Kinase Regulates Disseminated Prostate Cancer Cellular Dormancy.J Cell Biochem. 2017 Apr;118(4):891-902. doi: 10.1002/jcb.25768. Epub 2016 Nov 10. J Cell Biochem. 2017. PMID: 27753136 Free PMC article.
-
The biology and clinical implications of prostate cancer dormancy and metastasis.J Mol Med (Berl). 2016 Mar;94(3):259-65. doi: 10.1007/s00109-015-1353-4. Epub 2015 Oct 21. J Mol Med (Berl). 2016. PMID: 26489605 Free PMC article. Review.
-
AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications.Mol Cancer. 2019 Nov 4;18(1):153. doi: 10.1186/s12943-019-1090-3. Mol Cancer. 2019. PMID: 31684958 Free PMC article. Review.
Cited by
-
Metabolic Features of Tumor Dormancy: Possible Therapeutic Strategies.Cancers (Basel). 2022 Jan 21;14(3):547. doi: 10.3390/cancers14030547. Cancers (Basel). 2022. PMID: 35158815 Free PMC article. Review.
-
Dissecting the Role of AXL in Cancer Immune Escape and Resistance to Immune Checkpoint Inhibition.Front Immunol. 2022 Apr 27;13:869676. doi: 10.3389/fimmu.2022.869676. eCollection 2022. Front Immunol. 2022. PMID: 35572601 Free PMC article. Review.
-
Targeting rapid TKI-induced AXL upregulation overcomes adaptive ERK reactivation and exerts antileukemic effects in FLT3/ITD acute myeloid leukemia.Mol Oncol. 2025 May;19(5):1386-1403. doi: 10.1002/1878-0261.13749. Epub 2024 Oct 12. Mol Oncol. 2025. PMID: 39395205 Free PMC article.
-
Targeting AXL in NSCLC.Lung Cancer (Auckl). 2021 Aug 10;12:67-79. doi: 10.2147/LCTT.S305484. eCollection 2021. Lung Cancer (Auckl). 2021. PMID: 34408519 Free PMC article. Review.
-
Components of the Endosome-Lysosome Vesicular Machinery as Drivers of the Metastatic Cascade in Prostate Cancer.Cancers (Basel). 2024 Dec 26;17(1):43. doi: 10.3390/cancers17010043. Cancers (Basel). 2024. PMID: 39796673 Free PMC article. Review.
References
-
- Prostate Cancer: Statistics. Vol. 2018. (ASCO, Cancer.Net).
-
- Pound CR, Partin AW, Epstein JI & Walsh PC. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am. 1997;24:395–406. - PubMed
-
- Norgaard M, Jensen AO, Jacobsen JB, Cetin K, Fryzek JP & Sorensen HT. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007). J Urol. 2010;184:162–167. - PubMed
-
- Willis RA. The Spread of Tumours in the Human Body. London: J&A: Churchill; 1934;
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

