Dual Porosity Protein-based Scaffolds with Enhanced Cell Infiltration and Proliferation
- PMID: 30291271
- PMCID: PMC6173780
- DOI: 10.1038/s41598-018-33245-w
Dual Porosity Protein-based Scaffolds with Enhanced Cell Infiltration and Proliferation
Abstract
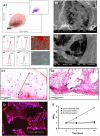
3D dual porosity protein-based scaffolds have been developed using the combination of foaming and freeze-drying. The suggested approach leads to the production of large, highly porous scaffolds with negligible shrinkage and deformation compared to the conventional freeze-drying method. Scanning electron microscopy, standard histological processing and mercury intrusion porosimetry confirmed the formation of a dual network in the form of big primary pores (243 ± 14 µm) embracing smaller secondary pores (42 ± 3 µm) opened onto their surface, resembling a vascular network. High interconnectivity of the pores, confirmed by micro-CT, is shown to improve diffusion kinetics and support a relatively uniform distribution of isolated human dental pulp stem cells within the scaffold compared to conventional scaffolds. Dual network scaffolds indicate more than three times as high cell proliferation capability as conventional scaffolds in 14 days.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Preparation of 3-D regenerated fibroin scaffolds with freeze drying method and freeze drying/foaming technique.J Mater Sci Mater Med. 2006 Dec;17(12):1349-56. doi: 10.1007/s10856-006-0610-z. J Mater Sci Mater Med. 2006. PMID: 17143767
-
Mechanisms of pore formation in hydrogel scaffolds textured by freeze-drying.Acta Biomater. 2019 Aug;94:195-203. doi: 10.1016/j.actbio.2019.05.070. Epub 2019 May 30. Acta Biomater. 2019. PMID: 31154055
-
Improvement of cell deposition by self-absorbent capability of freeze-dried 3D-bioprinted scaffolds derived from cellulose material-alginate hydrogels.Biomed Phys Eng Express. 2020 May 14;6(4):045009. doi: 10.1088/2057-1976/ab8fc6. Biomed Phys Eng Express. 2020. PMID: 33444270
-
Biphasic calcium phosphate scaffolds with controlled pore size distribution prepared by in-situ foaming.Mater Sci Eng C Mater Biol Appl. 2019 Feb 1;95:363-370. doi: 10.1016/j.msec.2018.03.022. Epub 2018 Mar 23. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 30573260
-
Engineering porous scaffolds using gas-based techniques.Curr Opin Biotechnol. 2011 Oct;22(5):661-6. doi: 10.1016/j.copbio.2011.04.005. Epub 2011 May 3. Curr Opin Biotechnol. 2011. PMID: 21546240 Review.
Cited by
-
Development of fibroblast/endothelial cell-seeded collagen scaffolds for in vitro prevascularization.J Biomed Mater Res B Appl Biomater. 2023 Mar;111(3):633-645. doi: 10.1002/jbm.b.35182. Epub 2022 Oct 19. J Biomed Mater Res B Appl Biomater. 2023. PMID: 36262080 Free PMC article.
-
Interface-Mediated Neurogenic Signaling: The Impact of Surface Geometry and Chemistry on Neural Cell Behavior for Regenerative and Brain-Machine Interfacing Applications.Adv Mater. 2024 Aug;36(33):e2401750. doi: 10.1002/adma.202401750. Epub 2024 Jul 3. Adv Mater. 2024. PMID: 38961531 Free PMC article. Review.
-
Stimuli-responsive hydrogels for manipulation of cell microenvironment: From chemistry to biofabrication technology.Prog Polym Sci. 2019 Nov;98:101147. doi: 10.1016/j.progpolymsci.2019.101147. Epub 2019 Jul 12. Prog Polym Sci. 2019. PMID: 36467305 Free PMC article.
-
Plant Cellulose as a Substrate for 3D Neural Stem Cell Culture.Bioengineering (Basel). 2023 Nov 13;10(11):1309. doi: 10.3390/bioengineering10111309. Bioengineering (Basel). 2023. PMID: 38002433 Free PMC article.
-
Screening of Self-Assembling of Collagen IV Fragments into Stable Structures Potentially Useful in Regenerative Medicine.Int J Mol Sci. 2021 Dec 18;22(24):13584. doi: 10.3390/ijms222413584. Int J Mol Sci. 2021. PMID: 34948383 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

