Structural insights into pro-aggregation effects of C. elegans CRAM-1 and its human ortholog SERF2
- PMID: 30291272
- PMCID: PMC6173753
- DOI: 10.1038/s41598-018-33143-1
Structural insights into pro-aggregation effects of C. elegans CRAM-1 and its human ortholog SERF2
Abstract
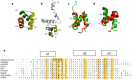
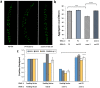
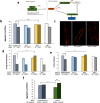
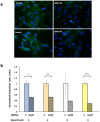
Toxic protein aggregates are key features of progressive neurodegenerative diseases. In addition to "seed" proteins diagnostic for each neuropathy (e.g., Aβ1-42 and tau in Alzheimer's disease), aggregates contain numerous other proteins, many of which are common to aggregates from diverse diseases. We reported that CRAM-1, discovered in insoluble aggregates of C. elegans expressing Q40::YFP, blocks proteasomal degradation of ubiquitinated proteins and thus promotes aggregation. We now show that CRAM-1 contains three α-helical segments forming a UBA-like domain, structurally similar to those of mammalian adaptor proteins (e.g. RAD23, SQSTM1/p62) that shuttle ubiquitinated cargos to proteasomes or autophagosomes for degradation. Molecular modeling indicates that CRAM-1, through this UBA-like domain, can form tight complexes with mono- and di-ubiquitin and may thus prevent tagged proteins from interacting with adaptor/shuttle proteins required for degradation. A human ortholog of CRAM-1, SERF2 (also largely disordered), promotes aggregation in SH-SY5Y-APPSw human neuroblastoma cells, since SERF2 knockdown protects these cells from amyloid formation. Atomistic molecular-dynamic simulations predict spontaneous unfolding of SERF2, and computational large-scale protein-protein interactions predict its stable binding to ubiquitins. SERF2 is also predicted to bind to most proteins screened at random, although with lower average stability than to ubiquitins, suggesting roles in aggregation initiation and/or progression.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Proteins in aggregates functionally impact multiple neurodegenerative disease models by forming proteasome-blocking complexes.Aging Cell. 2015 Feb;14(1):35-48. doi: 10.1111/acel.12296. Epub 2014 Dec 16. Aging Cell. 2015. PMID: 25510159 Free PMC article.
-
The cellular modifier MOAG-4/SERF drives amyloid formation through charge complementation.EMBO J. 2021 Nov 2;40(21):e107568. doi: 10.15252/embj.2020107568. Epub 2021 Oct 7. EMBO J. 2021. PMID: 34617299 Free PMC article.
-
Identification of MOAG-4/SERF as a regulator of age-related proteotoxicity.Cell. 2010 Aug 20;142(4):601-12. doi: 10.1016/j.cell.2010.07.020. Cell. 2010. PMID: 20723760
-
The UNC-45 myosin chaperone: from worms to flies to vertebrates.Int Rev Cell Mol Biol. 2014;313:103-44. doi: 10.1016/B978-0-12-800177-6.00004-9. Int Rev Cell Mol Biol. 2014. PMID: 25376491 Free PMC article. Review.
-
Degrade to create: developmental requirements for ubiquitin-mediated proteolysis during early C. elegans embryogenesis.Development. 2006 Mar;133(5):773-84. doi: 10.1242/dev.02276. Development. 2006. PMID: 16469970 Review.
Cited by
-
Glial Fibrillary Acidic Protein: A Biomarker and Drug Target for Alzheimer's Disease.Pharmaceutics. 2022 Jun 26;14(7):1354. doi: 10.3390/pharmaceutics14071354. Pharmaceutics. 2022. PMID: 35890250 Free PMC article.
-
Targeting C21orf58 is a Novel Treatment Strategy of Hepatocellular Carcinoma by Disrupting the Formation of JAK2/C21orf58/STAT3 Complex.Adv Sci (Weinh). 2024 Apr;11(15):e2306623. doi: 10.1002/advs.202306623. Epub 2024 Feb 11. Adv Sci (Weinh). 2024. PMID: 38342622 Free PMC article.
-
Intrinsically disordered proteins identified in the aggregate proteome serve as biomarkers of neurodegeneration.Metab Brain Dis. 2022 Jan;37(1):147-152. doi: 10.1007/s11011-021-00791-8. Epub 2021 Aug 4. Metab Brain Dis. 2022. PMID: 34347206 Free PMC article. Review.
-
Machine-learning analysis of intrinsically disordered proteins identifies key factors that contribute to neurodegeneration-related aggregation.Front Aging Neurosci. 2022 Aug 3;14:938117. doi: 10.3389/fnagi.2022.938117. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35992603 Free PMC article.
-
A Novel Microtubule-Binding Drug Attenuates and Reverses Protein Aggregation in Animal Models of Alzheimer's Disease.Front Mol Neurosci. 2019 Dec 12;12:310. doi: 10.3389/fnmol.2019.00310. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31920540 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

