Developmental asynchrony and antagonism of sex determination pathways in a lizard with temperature-induced sex reversal
- PMID: 30291276
- PMCID: PMC6173690
- DOI: 10.1038/s41598-018-33170-y
Developmental asynchrony and antagonism of sex determination pathways in a lizard with temperature-induced sex reversal
Abstract
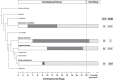
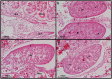
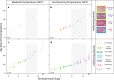
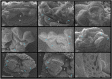
Vertebrate sex differentiation follows a conserved suite of developmental events: the bipotential gonads differentiate and shortly thereafter sex specific traits become dimorphic. However, this may not apply to squamates, a diverse vertebrate lineage comprising of many species with thermosensitive sexual development. Of the three species with data on the relative timing of gonad differentiation and genital dimorphism, the females of two (Niveoscincus ocellatus and Barisia imbricata) exhibit a phase of temporary pseudohermaphroditism or TPH (gonads have differentiated well before genital dimorphism). We report a third example of TPH in Pogona vitticeps, an agamid with temperature-induced male to female sex reversal. These findings suggest that for female squamates, genital and gonad development may not be closely synchronised, so that TPH may be common. We further observed a high frequency of ovotestes, a usually rare gonadal phenotype characterised by a mix of male and female structures, exclusively associated with temperature-induced sex reversal. We propose that ovotestes are evidence of a period of antagonism between male and female sex-determining pathways during sex reversal. Female sexual development in squamates is considerably more complex than has been appreciated, providing numerous avenues for future exploration of the genetic and hormonal cues that govern sexual development.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Ovotestes suggest cryptic genetic influence in a reptile model for temperature-dependent sex determination.Proc Biol Sci. 2021 Jan 27;288(1943):20202819. doi: 10.1098/rspb.2020.2819. Epub 2021 Jan 20. Proc Biol Sci. 2021. PMID: 33467998 Free PMC article.
-
Developmental dynamics of sex reprogramming by high incubation temperatures in a dragon lizard.BMC Genomics. 2022 Apr 22;23(1):322. doi: 10.1186/s12864-022-08544-2. BMC Genomics. 2022. PMID: 35459109 Free PMC article.
-
Embryonic gonadal and sexual organ development in a small viviparous skink, Niveoscincus ocellatus.J Exp Zool A Comp Exp Biol. 2006 Jan 1;305(1):74-82. doi: 10.1002/jez.a.249. J Exp Zool A Comp Exp Biol. 2006. PMID: 16358273
-
In search of determinants: gene expression during gonadal sex differentiation.J Fish Biol. 2010 May;76(8):1879-902. doi: 10.1111/j.1095-8649.2010.02594.x. J Fish Biol. 2010. PMID: 20557645 Review.
-
Temperature-Induced Sex Reversal in Reptiles: Prevalence, Discovery, and Evolutionary Implications.Sex Dev. 2021;15(1-3):148-156. doi: 10.1159/000515687. Epub 2021 Jun 10. Sex Dev. 2021. PMID: 34111872 Review.
Cited by
-
Two transcriptionally distinct pathways drive female development in a reptile with both genetic and temperature dependent sex determination.PLoS Genet. 2021 Apr 15;17(4):e1009465. doi: 10.1371/journal.pgen.1009465. eCollection 2021 Apr. PLoS Genet. 2021. PMID: 33857129 Free PMC article.
-
Gene expression of male pathway genes sox9 and amh during early sex differentiation in a reptile departs from the classical amniote model.BMC Genomics. 2023 May 5;24(1):243. doi: 10.1186/s12864-023-09334-0. BMC Genomics. 2023. PMID: 37147622 Free PMC article.
-
Ovotestes suggest cryptic genetic influence in a reptile model for temperature-dependent sex determination.Proc Biol Sci. 2021 Jan 27;288(1943):20202819. doi: 10.1098/rspb.2020.2819. Epub 2021 Jan 20. Proc Biol Sci. 2021. PMID: 33467998 Free PMC article.
-
Histological Analysis of Gonadal Ridge Development and Sex Differentiation of Gonads in Three Gecko Species.Biology (Basel). 2023 Dec 22;13(1):7. doi: 10.3390/biology13010007. Biology (Basel). 2023. PMID: 38248438 Free PMC article.
-
Developmental dynamics of sex reprogramming by high incubation temperatures in a dragon lizard.BMC Genomics. 2022 Apr 22;23(1):322. doi: 10.1186/s12864-022-08544-2. BMC Genomics. 2022. PMID: 35459109 Free PMC article.
References
-
- Wake, M. H. Hyman’s Comparative Vertebrate Anatomy. (The University of Chicago Press, 1992).
-
- Wibbels T, Wilson C, Crews D. Mullerian duct development andregression in a turtle with temperature-dependent sex determination. J. Herpetol. 1999;33:149–152. doi: 10.2307/1565558. - DOI
-
- Norris, D. L. K. Hormones and reproduction of vertebrates reptiles. Vol. 3 (Elsevier Science, 2011).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

