Empagliflozin Ammeliorates High Glucose Induced-Cardiac Dysfuntion in Human iPSC-Derived Cardiomyocytes
- PMID: 30291295
- PMCID: PMC6173708
- DOI: 10.1038/s41598-018-33293-2
Empagliflozin Ammeliorates High Glucose Induced-Cardiac Dysfuntion in Human iPSC-Derived Cardiomyocytes
Abstract
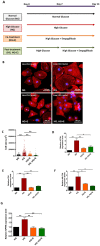
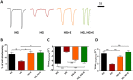
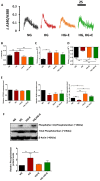
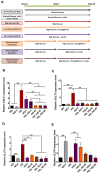
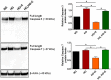
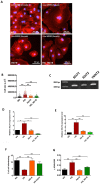
Empagliflozin, a sodium-glucose co-transporter (SGLT) inhibitor, reduces heart failure and sudden cardiac death but the underlying mechanisms remain elusive. In cardiomyocytes, SGLT1 and SGLT2 expression is upregulated in diabetes mellitus, heart failure, and myocardial infarction. We hypothesise that empagliflozin exerts direct effects on cardiomyocytes that attenuate diabetic cardiomyopathy. To test this hypothesis, cardiomyocytes derived from human induced pluripotent stem cells (hiPSCs) were used to test the potential effects of empagliflozin on neutralization of cardiac dysfunction induced by diabetic-like cultures. Our results indicated that insulin-free high glucose culture significantly increased the size of and NPPB, SGLT1 and SGLT2 expression of hiPSC-derived cardiomyocytes. In addition, high glucose-treated hiPSC-derived cardiomyocytes exhibited reduced contractility regardless of the increased calcium transient capacity. Interestingly, application of empagliflozin before or after high glucose treatment effectively reduced the high glucose-induced cardiac abnormalities. Since application of empagliflozin did not significantly alter viability or glycolytic capacity of the hiPSC-derived cardiomyocytes, it is plausible that empagliflozin exerts its effects via the down-regulation of SGLT1, SGLT2 and GLUT1 expression. These observations provide supportive evidence that may help explain its unexpected benefit observed in the EMPA-REG trial.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Empagliflozin Reduces High Glucose-Induced Cardiomyopathy in hiPSC-Derived Cardiomyocytes : Glucose-induced Lipotoxicity in hiPSC-Derived Cardiomyocytes.Stem Cell Rev Rep. 2025 Apr;21(3):849-858. doi: 10.1007/s12015-024-10839-8. Epub 2025 Jan 22. Stem Cell Rev Rep. 2025. PMID: 39841369
-
Long-term effects of empagliflozin on excitation-contraction-coupling in human induced pluripotent stem cell cardiomyocytes.J Mol Med (Berl). 2020 Dec;98(12):1689-1700. doi: 10.1007/s00109-020-01989-6. Epub 2020 Oct 9. J Mol Med (Berl). 2020. PMID: 33034709 Free PMC article.
-
Empagliflozin improves left ventricular diastolic function of db/db mice.Biochim Biophys Acta Mol Basis Dis. 2020 Aug 1;1866(8):165807. doi: 10.1016/j.bbadis.2020.165807. Epub 2020 Apr 28. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 32353614
-
SGLT2 inhibitors and cardioprotection: a matter of debate and multiple hypotheses.Postgrad Med. 2019 Mar;131(2):82-88. doi: 10.1080/00325481.2019.1581971. Postgrad Med. 2019. PMID: 30757937 Review.
-
Which is the main molecular target responsible for the cardiovascular benefits in the EMPA-REG OUTCOME trial? A journey through the kidney, the heart and other interesting places.Nutr Metab Cardiovasc Dis. 2016 Dec;26(12):1071-1078. doi: 10.1016/j.numecd.2016.09.001. Epub 2016 Sep 10. Nutr Metab Cardiovasc Dis. 2016. PMID: 27776917 Review.
Cited by
-
The dawn of the four-drug era? SGLT2 inhibition in heart failure with reduced ejection fraction.Ther Adv Cardiovasc Dis. 2021 Jan-Dec;15:17539447211002678. doi: 10.1177/17539447211002678. Ther Adv Cardiovasc Dis. 2021. PMID: 33779401 Free PMC article. Review.
-
Crosstalk between Sodium-Glucose Cotransporter Inhibitors and Sodium-Hydrogen Exchanger 1 and 3 in Cardiometabolic Diseases.Int J Mol Sci. 2021 Nov 24;22(23):12677. doi: 10.3390/ijms222312677. Int J Mol Sci. 2021. PMID: 34884494 Free PMC article. Review.
-
Metabolic and Mitochondrial Dysregulations in Diabetic Cardiac Complications.Int J Mol Sci. 2025 Mar 26;26(7):3016. doi: 10.3390/ijms26073016. Int J Mol Sci. 2025. PMID: 40243689 Free PMC article. Review.
-
Empagliflozin activates JAK2/STAT3 signaling and protects cardiomyocytes from hypoxia/reoxygenation injury under high glucose conditions.J Thromb Thrombolysis. 2023 Jan;55(1):116-125. doi: 10.1007/s11239-022-02719-0. Epub 2022 Nov 17. J Thromb Thrombolysis. 2023. PMID: 36396837
-
Transient receptor potential vanilloid type 1: cardioprotective effects in diabetic models.Channels (Austin). 2023 Dec;17(1):2281743. doi: 10.1080/19336950.2023.2281743. Epub 2023 Nov 20. Channels (Austin). 2023. PMID: 37983306 Free PMC article. Review.
References
-
- Steven Sebastian, Oelze Matthias, Hanf Alina, Kröller-Schön Swenja, Kashani Fatemeh, Roohani Siyer, Welschof Philipp, Kopp Maximilian, Gödtel-Armbrust Ute, Xia Ning, Li Huige, Schulz Eberhard, Lackner Karl J., Wojnowski Leszek, Bottari Serge P., Wenzel Philip, Mayoux Eric, Münzel Thomas, Daiber Andreas. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biology. 2017;13:370–385. doi: 10.1016/j.redox.2017.06.009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

