Temporal differentiation of bovine airway epithelial cells grown at an air-liquid interface
- PMID: 30291311
- PMCID: PMC6173764
- DOI: 10.1038/s41598-018-33180-w
Temporal differentiation of bovine airway epithelial cells grown at an air-liquid interface
Abstract
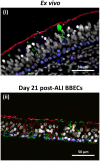
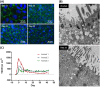
There is an urgent need to develop improved, physiologically-relevant in vitro models of airway epithelia with which to better understand the pathological processes associated with infection, allergies and toxicological insults of the respiratory tract of both humans and domesticated animals. In the present study, we have characterised the proliferation and differentiation of primary bovine bronchial epithelial cells (BBECs) grown at an air-liquid interface (ALI) at three-day intervals over a period of 42 days from the introduction of the ALI. The differentiated BBEC model was highly representative of the ex vivo epithelium from which the epithelial cells were derived; a columnar, pseudostratified epithelium that was highly reflective of native airway epithelium was formed which comprised ciliated, goblet and basal cells. The hallmark defences of the respiratory tract, namely barrier function and mucociliary clearance, were present, thus demonstrating that the model is an excellent mimic of bovine respiratory epithelium. The epithelium was fully differentiated by day 21 post-ALI and, crucially, remained healthy and stable for a further 21 days. Thus, the differentiated BBEC model has a three-week window which will allow wide-ranging and long-term experiments to be performed in the fields of infection, toxicology or general airway physiology.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Development and optimization of a differentiated airway epithelial cell model of the bovine respiratory tract.Sci Rep. 2018 Jan 16;8(1):853. doi: 10.1038/s41598-017-19079-y. Sci Rep. 2018. PMID: 29339818 Free PMC article.
-
Structural and functional variations in human bronchial epithelial cells cultured in air-liquid interface using different growth media.Am J Physiol Lung Cell Mol Physiol. 2020 May 1;318(5):L1063-L1073. doi: 10.1152/ajplung.00190.2019. Epub 2020 Mar 25. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32208929
-
Tissue-Specific stem cell differentiation in an in vitro airway model.Macromol Biosci. 2011 Nov 10;11(11):1467-77. doi: 10.1002/mabi.201100181. Epub 2011 Oct 12. Macromol Biosci. 2011. PMID: 21994115
-
Air-liquid interface cell culture: From airway epithelium to the female reproductive tract.Reprod Domest Anim. 2019 Sep;54 Suppl 3:38-45. doi: 10.1111/rda.13481. Reprod Domest Anim. 2019. PMID: 31512315 Review.
-
Air-liquid interface (ALI) impact on different respiratory cell cultures.Eur J Pharm Biopharm. 2023 Mar;184:62-82. doi: 10.1016/j.ejpb.2023.01.013. Epub 2023 Jan 22. Eur J Pharm Biopharm. 2023. PMID: 36696943 Review.
Cited by
-
Mannheimia haemolytica and lipopolysaccharide induce airway epithelial inflammatory responses in an extensively developed ex vivo calf model.Sci Rep. 2020 Aug 3;10(1):13042. doi: 10.1038/s41598-020-69982-0. Sci Rep. 2020. PMID: 32747652 Free PMC article.
-
Rock Inhibitor Y-27632 Enables Feeder-Free, Unlimited Expansion of Sus scrofa domesticus Swine Airway Stem Cells to Facilitate Respiratory Research.Stem Cells Int. 2019 Nov 23;2019:3010656. doi: 10.1155/2019/3010656. eCollection 2019. Stem Cells Int. 2019. PMID: 31871466 Free PMC article.
-
The Cell Tropism of Porcine Respiratory Coronavirus for Airway Epithelial Cells Is Determined by the Expression of Porcine Aminopeptidase N.Viruses. 2020 Oct 23;12(11):1211. doi: 10.3390/v12111211. Viruses. 2020. PMID: 33114247 Free PMC article.
-
Generation of Human Endometrial Assembloids with a Luminal Epithelium using Air-Liquid Interface Culture Methods.Adv Sci (Weinh). 2023 Oct;10(30):e2301868. doi: 10.1002/advs.202301868. Epub 2023 Aug 27. Adv Sci (Weinh). 2023. PMID: 37635169 Free PMC article.
-
The Impact of NO2 on Epithelial Barrier Integrity of a Primary Cell-Based Air-Liquid Interface Model of the Nasal Respiratory Epithelium.J Appl Toxicol. 2025 Mar;45(3):482-491. doi: 10.1002/jat.4717. Epub 2024 Nov 12. J Appl Toxicol. 2025. PMID: 39529574 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

