FDG PET/CT for assessing tumour response to immunotherapy : Report on the EANM symposium on immune modulation and recent review of the literature
- PMID: 30291373
- PMCID: PMC6267687
- DOI: 10.1007/s00259-018-4171-4
FDG PET/CT for assessing tumour response to immunotherapy : Report on the EANM symposium on immune modulation and recent review of the literature
Abstract
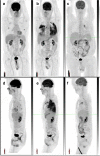
This paper follows the immunotherapy symposium held during the European Association of Nuclear Medicine (EANM) 2017 Annual Congress. The biological basis of the immune checkpoint inhibitors and the drugs most frequently used for the treatment of solid tumours are reviewed. The issues of pseudoprogression (frequency, timeline), hyperprogression and immune-related side effects are discussed, as well as their implications for patient management. A review of the recent literature on the use of FDG PET for assessment of immunotherapy is presented, and recommendations are provided for assessing tumour response and reporting immune-related side effects with FDG PET based on published data and experts' experience. Representative clinical cases are also discussed.
Keywords: Hyperprogression; Immune checkpoint inhibitors; Immune-related side effects; Immunotherapy; Pseudoprogression; Therapy response.
Conflict of interest statement
Conflicts of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
In accordance with European regulations, written consent was obtained from all patients to use their anonymized PET images.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

