Clinical factors predicting treatment resistant depression: affirmative results from the European multicenter study
- PMID: 30291625
- PMCID: PMC6586002
- DOI: 10.1111/acps.12959
Clinical factors predicting treatment resistant depression: affirmative results from the European multicenter study
Abstract
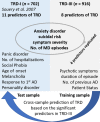
Objectives: Clinical variables were investigated in the 'treatment resistant depression (TRD)- III' sample to replicate earlier findings by the European research consortium 'Group for the Study of Resistant Depression' (GSRD) and enable cross-sample prediction of treatment outcome in TRD.
Experimental procedures: TRD was defined by a Montgomery and Åsberg Depression Rating Scale (MADRS) score ≥22 after at least two antidepressive trials. Response was defined by a decline in MADRS score by ≥50% and below a threshold of 22. Logistic regression was applied to replicate predictors for TRD among 16 clinical variables in 916 patients. Elastic net regression was applied for prediction of treatment outcome.
Results: Symptom severity (odds ratio (OR) = 3.31), psychotic symptoms (OR = 2.52), suicidal risk (OR = 1.74), generalized anxiety disorder (OR = 1.68), inpatient status (OR = 1.65), higher number of antidepressants administered previously (OR = 1.23), and lifetime depressive episodes (OR = 1.15) as well as longer duration of the current episode (OR = 1.022) increased the risk of TRD. Prediction of TRD reached an accuracy of 0.86 in the independent validation set, TRD-I.
Conclusion: Symptom severity, suicidal risk, higher number of lifetime depressive episodes, and comorbid anxiety disorder were replicated as the most prominent risk factors for TRD. Significant predictors in TRD-III enabled robust prediction of treatment outcome in TRD-I.
Keywords: antidepressives; clinical aspects; depression.
© 2018 The Authors Acta Psychiatrica Scandinavica Published by John Wiley & Sons Ltd.
Conflict of interest statement
The Group for the Study of Resistant Depression (GRSD) was supported by an unrestricted grant from Lundbeck that had no further role in the study design, data collection, analysis and interpretation, as well as in the writing and submitting of the manuscript for publication. Dr. Dold has received a travel grant from Janssen‐Cilag. Dr. Kasper received grants/research support, consulting fees and/or honoraria within the last 3 years from Angelini, AOP Orphan Pharmaceuticals AG, AstraZeneca, Eli Lilly, Janssen, KRKA‐Pharma, Lundbeck, Neuraxpharm, Pfizer, Pierre Fabre, Schwabe, and Servier. Dr. Souery has received grant/research support from GlaxoSmithKline and Lundbeck, and he has served as a consultant or on advisory boards for AstraZeneca, Bristol‐Myers Squibb, Eli Lilly, Janssen, and Lundbeck. Dr. Mendlewicz is a member of the board of the Lundbeck International Neuroscience Foundation and of the advisory board of Servier. Dr. Lanzenberger received travel grants and/or conference speaker honoraria from Shire, AstraZeneca, Lundbeck A/S, Dr. Willmar Schwabe GmbH, Orphan Pharmaceuticals AG, Janssen‐Cilag Pharma GmbH, and Roche Austria GmbH. Dr. Serretti is or has been consultant/speaker for Abbott, Abbvie, Angelini, AstraZeneca, Clinical Data, Boheringer, Bristol‐Myers Squibb, Eli Lilly, GlaxoSmithKline, Innovapharma, Italfarmaco, Janssen, Lundbeck, Naurex, Pfizer, Polifarma, Sanofi, and Servier. Dr. Zohar has received grant/research support from Lundbeck, Servier, and Pfizer; he has served as a consultantor on the advisory boards for Servier, Pfizer, Solvay, and Actelion; and he has served on speakers’ bureaus for Lundbeck, GSK, Jazz, and Solvay. Dr. Montgomery has been a consultant or served on advisory boards for AstraZeneca, Bionevia, Bristol‐Myers Squibb, Forest, GlaxoSmithKline, Grunenthal, Intellect Pharma, Johnson & Johnson, Lilly, Lundbeck, Merck, Merz, M's Science, Neurim, Otsuka, Pierre Fabre, Pfizer, Pharmaneuroboost, Richter, Roche, Sanofi, Sepracor, Servier, Shire, Synosis, Takeda, Theracos, Targacept, Transcept, UBC, Xytis, and Wyeth. All other authors declare that they have no conflict of interests.
Figures
References
-
- WHO . World Health Report: mental health – new understanding, new hope. Geneva: WHO; 2001: p 2001.
-
- Whiteford HA, Degenhardt L, Rehm J et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 2013;382:1575–1586. - PubMed
-
- Perlis RH. Pharmacogenomic testing and personalized treatment of depression. Clin Chem 2014;60:53–59. - PubMed
-
- Schosser A, Serretti A, Souery D et al. European Group for the Study of Resistant Depression (GSRD)–where have we gone so far: review of clinical and genetic findings. Eur Neuropsychopharmacol 2012;22:453–468. - PubMed
-
- Dold M, Kasper S. Evidence‐based pharmacotherapy of treatment‐resistant unipolar depression. Int J Psychiatry Clin Pract 2017;21:1–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

