Alkyl Carbon-Carbon Bond Formation by Nickel/Photoredox Cross-Coupling
- PMID: 30291664
- PMCID: PMC6551614
- DOI: 10.1002/anie.201809431
Alkyl Carbon-Carbon Bond Formation by Nickel/Photoredox Cross-Coupling
Abstract
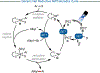
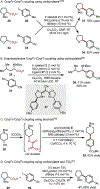
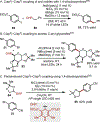
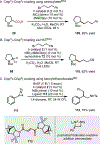
The union of photoredox and nickel catalysis has resulted in a renaissance in radical chemistry as well as in the use of nickel-catalyzed transformations, specifically for carbon-carbon bond formation. Collectively, these advances address the longstanding challenge of late-stage cross-coupling of functionalized alkyl fragments. Empowered by the notion that photocatalytically generated alkyl radicals readily undergo capture by Ni complexes, wholly new feedstocks for cross-coupling have been realized. Herein, we highlight recent developments in several types of alkyl cross-couplings that are accessible exclusively through this approach.
Keywords: alkyl radicals; cross-coupling; nickel; photocatalysis; single-electron transmetalation.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures


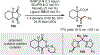
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

