A prospective comparison of cancer clinical trial availability and enrollment among adolescents/young adults treated at an adult cancer hospital or affiliated children's hospital
- PMID: 30291804
- PMCID: PMC6234084
- DOI: 10.1002/cncr.31727
A prospective comparison of cancer clinical trial availability and enrollment among adolescents/young adults treated at an adult cancer hospital or affiliated children's hospital
Abstract
Background: Low cancer clinical trial (CCT) enrollment may contribute to survival disparities affecting adolescents and young adults (AYAs) (ages 15-39 years). The objective of this study was to evaluate whether differences in CCT availability related to treatment site could explain the low CCT enrollment.
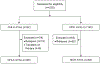
Methods: This prospective, observational cohort study was conducted at an academic children's hospital and its affiliated but geographically separated adult cancer hospital within a National Cancer Institute-designated Comprehensive Cancer Center. For consecutive, newly diagnosed AYA patients, it was determined whether an appropriate CCT existed nationally, was available at the treatment site, and was used for enrollment. Proportions of AYAs in these categories were compared between sites using the chi-square test.
Results: One hundred fifty-two consecutive AYA patients were included from the children's hospital (n = 68; ages 15-20 years) and the adult cancer hospital (n = 84; ages 18-39 years). Although there was no difference in CCT existence for individual AYA patients by site (children's hospital [36 of 68 patients; 52.9%] vs adult cancer hospital [45 of 84 patients; 53.6%]; P = .938), CCT availability was significantly lower at the adult cancer hospital (14 of 84 patients [16.7%] vs 30 of 68 [44.1%] at the children's hospital; P < .001). The proportion of AYAs enrolled was low at both sites (8 of 68 patients [11.8%] vs 6 of 84 patients [7.1%], respectively; P = .327). Fewer existing CCTs were available at the adult cancer hospital (4 of 27 patients [14.8%] vs 8 of 14 patients [57.1%], respectively), and those were directed toward solid tumors and new agents.
Conclusions: Efforts to improve low CCT enrollment among AYAs should be differentiated by treatment site. In the adult setting, these efforts should be aimed at improving CCT availability by overcoming site-level barriers to opening existing CCTs.
Keywords: adolescent; adolescents and young adults (AYAs); clinical oncology; clinical trial as topic; multicenter studies as topic; pediatric oncology; young adult.
© 2018 American Cancer Society.
Conflict of interest statement
Figures

Similar articles
-
A prospective, observational cohort study comparing cancer clinical trial availability and enrollment between early adolescents/young adults and children.Cancer. 2018 Mar 1;124(5):983-990. doi: 10.1002/cncr.31127. Epub 2017 Nov 17. Cancer. 2018. PMID: 29149450 Free PMC article.
-
Enrollment of adolescents and young adults onto SWOG cancer research network clinical trials: A comparative analysis by treatment site and era.Cancer Med. 2020 Mar;9(6):2146-2152. doi: 10.1002/cam4.2891. Epub 2020 Feb 3. Cancer Med. 2020. PMID: 32009305 Free PMC article.
-
Case-linked analysis of clinical trial enrollment among adolescents and young adults at a National Cancer Institute-designated comprehensive cancer center.Cancer. 2015 Dec 15;121(24):4398-406. doi: 10.1002/cncr.29669. Epub 2015 Sep 22. Cancer. 2015. PMID: 26393950 Free PMC article.
-
Systematic review of barriers and facilitators to clinical trial enrollment among adolescents and young adults with cancer: Identifying opportunities for intervention.Cancer. 2020 Mar 1;126(5):949-957. doi: 10.1002/cncr.32675. Epub 2019 Dec 23. Cancer. 2020. PMID: 31869454 Free PMC article.
-
Dynamics and Challenges of Clinical Trials in Adolescents and Young Adults With Cancer.Cancer J. 2018 Nov/Dec;24(6):307-314. doi: 10.1097/PPO.0000000000000347. Cancer J. 2018. PMID: 30480575 Review.
Cited by
-
Barriers and Facilitators to Adolescent and Young Adult Cancer Trial Enrollment: NCORP Site Perspectives.JNCI Cancer Spectr. 2021 Mar 22;5(3):pkab027. doi: 10.1093/jncics/pkab027. eCollection 2021 Jun. JNCI Cancer Spectr. 2021. PMID: 34104866 Free PMC article.
-
Creation of a quality improvement collaborative to address adolescent and young adult cancer clinical trial enrollment: ATAQI (AYA trial access quality initiative).Curr Probl Cancer. 2023 Dec;47(6):100898. doi: 10.1016/j.currproblcancer.2022.100898. Epub 2022 Sep 28. Curr Probl Cancer. 2023. PMID: 36207194 Free PMC article.
-
Barriers to Pediatric Oncologist Enrollment of Adolescents and Young Adults on a Cross-Network National Clinical Trials Network Supportive Care Cancer Clinical Trial.J Adolesc Young Adult Oncol. 2022 Feb;11(1):117-121. doi: 10.1089/jayao.2021.0041. Epub 2021 May 13. J Adolesc Young Adult Oncol. 2022. PMID: 33983848 Free PMC article.
-
Challenges and limitations of clinical trials in the adolescent and young adult CNS cancer population: A systematic review.Neurooncol Adv. 2023 Dec 10;6(1):vdad159. doi: 10.1093/noajnl/vdad159. eCollection 2024 Jan-Dec. Neurooncol Adv. 2023. PMID: 38250563 Free PMC article.
-
Sharing is caring: a network collaborative approach to identify and address barriers in accessing clinical trials in adolescents and young adults with leukemia and lymphoma.Hematology Am Soc Hematol Educ Program. 2024 Dec 6;2024(1):27-33. doi: 10.1182/hematology.2024000526. Hematology Am Soc Hematol Educ Program. 2024. PMID: 39643982 Free PMC article. Review.
References
-
- Bleyer WA, Tejeda H, Murphy SB, et al. National cancer clinical trials: children have equal access; adolescents do not. J Adolesc Health. 1997;21(6):366–373. - PubMed
-
- National Cancer Institute LAF. Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer: Report of the Adolescent and Young Adult Oncology Progress Review Group. 2006.
-
- Bleyer A, Montello M, Budd T, Saxman S. National survival trends of young adults with sarcoma: lack of progress is associated with lack of clinical trial participation. Cancer. 2005;103(9):1891–1897. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

