Protein-based nanoparticles in cancer vaccine development
- PMID: 30291897
- PMCID: PMC6289732
- DOI: 10.1016/j.nano.2018.09.004
Protein-based nanoparticles in cancer vaccine development
Abstract
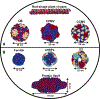
Peptide and protein-based cancer vaccines usually fail to elicit efficient immune responses against tumors. However, delivery of these peptides and proteins as components within caged protein nanoparticles has shown promising improvements in vaccine efficacy. Advantages of protein nanoparticles over other vaccine platforms include their highly organized structures and symmetry, biodegradability, ability to be specifically functionalized at three different interfaces (inside and outside the protein cage, and between subunits in macromolecular assembly), and ideal size for vaccine delivery. In this review, we discuss different classes of virus-like particles and caged protein nanoparticles that have been used as vehicles to transport and increase the interaction of cancer vaccine components with the immune system. We review the effectiveness of these protein nanoparticles towards inducing and elevating specific immune responses, which are needed to overcome the low immunogenicity of the tumor microenvironment.
Keywords: Caged protein nanoparticles; Cancer vaccines; Tumor antigens; Virus-like particles.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

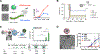
References
-
- Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat. Med. 2013; 19: 1597–1608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

