Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial
- PMID: 30292578
- PMCID: PMC6182127
- DOI: 10.1016/S0140-6736(18)31947-0
Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial
Erratum in
-
Department of Error.Lancet. 2018 Oct 13;392(10155):1310. doi: 10.1016/S0140-6736(18)32477-2. Lancet. 2018. PMID: 30322581 Free PMC article. No abstract available.
Abstract
Background: The achievement of glycaemic control remains challenging for patients with type 1 diabetes. We assessed the effectiveness of day-and-night hybrid closed-loop insulin delivery compared with sensor-augmented pump therapy in people with suboptimally controlled type 1 diabetes aged 6 years and older.
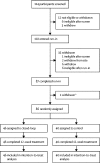
Methods: In this open-label, multicentre, multinational, single-period, parallel randomised controlled trial, participants were recruited from diabetes outpatient clinics at four hospitals in the UK and two centres in the USA. We randomly assigned participants with type 1 diabetes aged 6 years and older treated with insulin pump and with suboptimal glycaemic control (glycated haemoglobin [HbA1c] 7·5-10·0%) to receive either hybrid closed-loop therapy or sensor-augmented pump therapy over 12 weeks of free living. Training on study insulin pump and continuous glucose monitoring took place over a 4-week run-in period. Eligible subjects were randomly assigned using central randomisation software. Allocation to the two study groups was unblinded, and randomisation was stratified within centre by low (<8·5%) or high (≥8·5%) HbA1c. The primary endpoint was the proportion of time that glucose concentration was within the target range of 3·9-10·0 mmol/L at 12 weeks post randomisation. Analyses of primary outcome and safety measures were done in all randomised patients. The trial is registered with ClinicalTrials.gov, number NCT02523131, and is closed to accrual.
Findings: From May 12, 2016, to Nov 17, 2017, 114 individuals were screened, and 86 eligible patients were randomly assigned to receive hybrid closed-loop therapy (n=46) or sensor-augmented pump therapy (n=40; control group). The proportion of time that glucose concentration was within the target range was significantly higher in the closed-loop group (65%, SD 8) compared with the control group (54%, SD 9; mean difference in change 10·8 percentage points, 95% CI 8·2 to 13·5; p<0·0001). In the closed-loop group, HbA1c was reduced from a screening value of 8·3% (SD 0·6) to 8·0% (SD 0·6) after the 4-week run-in, and to 7·4% (SD 0·6) after the 12-week intervention period. In the control group, the HbA1c values were 8·2% (SD 0·5) at screening, 7·8% (SD 0·6) after run-in, and 7·7% (SD 0·5) after intervention; reductions in HbA1c percentages were significantly greater in the closed-loop group compared with the control group (mean difference in change 0·36%, 95% CI 0·19 to 0·53; p<0·0001). The time spent with glucose concentrations below 3·9 mmol/L (mean difference in change -0·83 percentage points, -1·40 to -0·16; p=0·0013) and above 10·0 mmol/L (mean difference in change -10·3 percentage points, -13·2 to -7·5; p<0·0001) was shorter in the closed-loop group than the control group. The coefficient of variation of sensor-measured glucose was not different between interventions (mean difference in change -0·4%, 95% CI -1·4% to 0·7%; p=0·50). Similarly, total daily insulin dose was not different (mean difference in change 0·031 U/kg per day, 95% CI -0·005 to 0·067; p=0·09) and bodyweight did not differ (mean difference in change 0·68 kg, 95% CI -0·34 to 1·69; p=0·19). No severe hypoglycaemia occurred. One diabetic ketoacidosis occurred in the closed-loop group due to infusion set failure. Two participants in each study group had significant hyperglycaemia, and there were 13 other adverse events in the closed-loop group and three in the control group.
Interpretation: Hybrid closed-loop insulin delivery improves glucose control while reducing the risk of hypoglycaemia across a wide age range in patients with suboptimally controlled type 1 diabetes.
Funding: JDRF, NIHR, and Wellcome Trust.
Copyright © 2018 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures


Comment in
-
Enlarging the loop: closed-loop insulin delivery for type 1 diabetes.Lancet. 2018 Oct 13;392(10155):1282-1284. doi: 10.1016/S0140-6736(18)32329-8. Epub 2018 Oct 3. Lancet. 2018. PMID: 30292579 No abstract available.
-
Closed-loop insulin delivery has wide-ranging benefits.Nat Rev Endocrinol. 2018 Dec;14(12):688. doi: 10.1038/s41574-018-0117-y. Nat Rev Endocrinol. 2018. PMID: 30341442 No abstract available.
References
-
- International Diabetes Federation IDF Diabetes Atlas. 2017. http://www.diabetesatlas.org/ 8th edn.
-
- Miller KM, Foster NC, Beck RW. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38:971–978. - PubMed
-
- UK Hypoglycaemia Study Group Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50:1140–1147. - PubMed
-
- Battelino T, Nimri R, Dovc K, Phillip M, Bratina N. Prevention of hypoglycemia with predictive low glucose insulin suspension in children with type 1 diabetes: a randomized controlled trial. Diabetes Care. 2017;40:764–770. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

