Erythropoietin-producing hepatocellular A7 triggering ovulation indicates a potential beneficial role for polycystic ovary syndrome
- PMID: 30292674
- PMCID: PMC6197718
- DOI: 10.1016/j.ebiom.2018.09.046
Erythropoietin-producing hepatocellular A7 triggering ovulation indicates a potential beneficial role for polycystic ovary syndrome
Abstract
Background: The ovulatory dysfunction mechanisms underlying polycystic ovary syndrome (PCOS) are not completely understood. And the roles of EPHA7 and EPHA7-regulated pathway factors in the pathogenesis of anovulation remain to be elucidated.
Methods: We used human granulosa cells (hGCs) of PCOS and non-PCOS patients to measure EPHA7 and other target gene expressions. We performed in vitro experiments in KGN cells to verify the molecular mechanisms. Additionally, we conducted in vivo loss- and gain-of-function studies using EPHA7 shRNA lentivirus and recombinant EPHA7-Fc protein injection to identify the ovulation effects of EPHA7.
Findings: EPHA7 functions as a critically positive upstream factor for the expression of ERK1/2-mediated C/EBPβ. This protein, in turn, induced the expression of KLF4 and then ADAMTS1. Moreover, decreased abundance of EPHA7 was positively correlated with that of its downstream factors in hGCs of PCOS patients. Additionally, a 1-week functional EPHA7 shRNA lentivirus in rat ovaries contributed to decreased numbers of retrieved oocytes, and a 3-week functional lentivirus led to menstrual disorders and morphological polycystic changes in rat ovaries. More importantly, we found that EPHA7 triggered ovulation in rats, and it improved polycystic ovarian changes induced by DHEA in PCOS rats.
Interpretation: Our findings demonstrate a new role of EPHA7 in PCOS, suggesting that EPHA7 is an effective target for the development of innovative medicines to induce ovulation. FUND: National Key Research and Development Program of China, National Natural Science Foundation, Shanghai Municipal Education Commission--Gaofeng Clinical Medicine, and Shanghai Commission of Science and Technology.
Keywords: EPHA7; Female fertility; Ovulation; PCOS.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



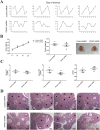

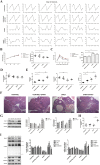
References
-
- Stepto N.K., Cassar S., Joham A.E., Hutchison S.K., Harrison C.L., Goldstein R.F. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod. 2013;28:777–784. - PubMed
-
- Franks S., Stark J., Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14:367–378. - PubMed
-
- Koskinen P., Penttila T.A., Anttila L., Erkkola R., Irjala K. Optimal use of hormone determinations in the biochemical diagnosis of the polycystic ovary syndrome. Fertil Steril. 1996;65:517–522. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

