A Randomized, Double-Blind, Placebo-Controlled Phase 3 Trial of Oral Brincidofovir for Cytomegalovirus Prophylaxis in Allogeneic Hematopoietic Cell Transplantation
- PMID: 30292744
- PMCID: PMC8196624
- DOI: 10.1016/j.bbmt.2018.09.038
A Randomized, Double-Blind, Placebo-Controlled Phase 3 Trial of Oral Brincidofovir for Cytomegalovirus Prophylaxis in Allogeneic Hematopoietic Cell Transplantation
Abstract
Cytomegalovirus (CMV) infection is a common complication of allogeneic hematopoietic cell transplantation (HCT). In this trial, we randomized adult CMV-seropositive HCT recipients without CMV viremia at screening 2:1 to receive brincidofovir or placebo until week 14 post-HCT. Randomization was stratified by center and risk of CMV infection. Patients were assessed weekly through week 15 and every third week thereafter through week 24 post-HCT. Patients who developed clinically significant CMV infection (CS-CMVi; CMV viremia requiring preemptive therapy or CMV disease) discontinued the study drug and began anti-CMV treatment. The primary endpoint was the proportion of patients with CS-CMVi through week 24 post-HCT; patients who discontinued the trial or with missing data were imputed as primary endpoint events. Between August 2013 and June 2015, 452 patients were randomized at a median of 15 days after HCT and received study drug. The proportion of patients who developed CS-CMVi or were imputed as having a primary endpoint event through week 24 was similar between brincidofovir-treated patients and placebo recipients (155 of 303 [51.2%] versus 78 of 149 [52.3%]; odds ratio, .95 [95% confidence interval, .64 to 1.41]; P = .805); fewer brincidofovir recipients developed CMV viremia through week 14 compared with placebo recipients (41.6%; P < .001). Serious adverse events were more frequent among brincidofovir recipients (57.1% versus 37.6%), driven by acute graft-versus-host disease (32.3% versus 6.0%) and diarrhea (6.9% versus 2.7%). Week 24 all-cause mortality was 15.5% among brincidofovir recipients and 10.1% among placebo recipients. Brincidofovir did not reduce CS-CMVi by week 24 post-HCT and was associated with gastrointestinal toxicity.
Keywords: Allogeneic hematopoietic cell transplantation; Antiviral; Brincidofovir; CMX001; Cytomegalovirus; Prophylaxis.
Copyright © 2018. Published by Elsevier Inc.
Figures


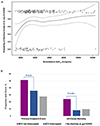
References
-
- Boeckh M, Nichols WG, Papanicolaou G, Rubin R, Wingard JR, Zaia J. Cytomegalovirus in hematopoietic stem cell transplant recipients: current status, known challenges, and future strategies. Biol Blood Marrow Transplant. 2003;9:543–558. - PubMed
-
- Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Infect Dis Clin North Am. 2010;24:319–337. - PubMed
-
- Craddock C, Szydlo RM, Dazzi F, et al. Cytomegalovirus seropositivity adversely influences outcome after T-depleted unrelated donor transplant in patients with chronic myeloid leukaemia: the case for tailored graft-versus-host disease prophylaxis. Br J Haematol. 2001;112:228–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

