Role of aberrant glycosylation enzymes in oral cancer progression
- PMID: 30294247
- PMCID: PMC6166416
- DOI: 10.4103/jcar.JCar_7_18
Role of aberrant glycosylation enzymes in oral cancer progression
Abstract
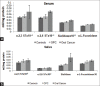
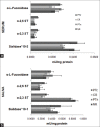
Background: Carcinogenesis, a multistep process involves sequential changes during neoplastic transformation. The various hallmarks of cancer aid in cell survival, proliferation, and dissemination. Aberrant glycosylation, a recently defined hallmark of cancer, is influenced by glycosylation enzymes during carcinogenesis. Therefore, the present study measured α-2,3 and α-2,6 sialyltransferase (ST), sialidase, and α-L-fucosidase activity in patients with oral precancerous conditions (OPC) and oral cancer patients.
Subjects: The study enrolled 100 oral cancer patients, 50 patients with OPC, 100 healthy controls, and 46 posttreatment follow-ups of oral cancer patients. Blood and saliva were collected from all the participants.
Materials and methods: Sialidase activity was measured by spectrofluorimetric method, α-2,3 and α-2,6 ST by ELISA using biotinylated lectins, and α-L-fucosidase by spectrophotometric method.
Results: The results depicted increased levels of sialidase, α-2,3 and α-2,6 ST, α-L-fucosidase in patients with OPC and oral cancer patients. Receiver operating characteristic curve indicated significant discriminatory efficacy in distinguishing controls and oral cancer patients for serum and salivary sialidase and α-L-fucosidase activity, and serum α-2,6 ST. Furthermore, serum and salivary α-L-fucosidase activity and serum sialidase activity significantly distinguished controls and patients with OPC. Serum and salivary sialidase, α-L-fucosidase, and serum α-2,3 ST activity were higher in patients with metastasis as compared to nonmetastatic patients. Higher values of serum α-L-fucosidase activity were significantly associated with low-overall survival.
Conclusion: The increased levels of enzymes correlated with tumor initiation, progression, and metastasis in oral cancer patients. The alterations in glycosyltransferases/glycosidases thus support the view of glycosylation as a hallmark of cancer.
Keywords: Fucosidase; fucosyltransferase; glycosylation; glycosyltransferase; hallmark; oral cancer; saliva; sialidase; sialyltransferase.
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
Salivary glyco-sialylation changes monitors oral carcinogenesis.Glycoconj J. 2014 Dec;31(9):649-59. doi: 10.1007/s10719-014-9561-7. Epub 2014 Oct 16. Glycoconj J. 2014. PMID: 25318700
-
Serum fucosylation changes in oral cancer and oral precancerous conditions: alpha-L-fucosidase as a marker.Cancer. 2008 Jul 15;113(2):336-46. doi: 10.1002/cncr.23556. Cancer. 2008. PMID: 18521898
-
Evaluation of serum and salivary total sialic acid and α-l-fucosidase in patients with oral precancerous conditions and oral cancer.Oral Surg Oral Med Oral Pathol Oral Radiol. 2013 Jun;115(6):764-71. doi: 10.1016/j.oooo.2013.01.004. Epub 2013 Apr 6. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013. PMID: 23570662
-
Glycosylation: a hallmark of cancer?Glycoconj J. 2017 Apr;34(2):147-156. doi: 10.1007/s10719-016-9755-2. Epub 2016 Dec 14. Glycoconj J. 2017. PMID: 27975160 Review.
-
Methods for improving enzymatic trans-glycosylation for synthesis of human milk oligosaccharide biomimetics.J Agric Food Chem. 2014 Oct 8;62(40):9615-31. doi: 10.1021/jf502619p. Epub 2014 Sep 23. J Agric Food Chem. 2014. PMID: 25208138 Review.
Cited by
-
Serum Sialylation Changes in Actinic Keratosis and Cutaneous Squamous Cell Carcinoma Patients.J Pers Med. 2021 Oct 15;11(10):1027. doi: 10.3390/jpm11101027. J Pers Med. 2021. PMID: 34683168 Free PMC article.
-
Sialidase and Sialyltransferase Inhibitors: Targeting Pathogenicity and Disease.Front Mol Biosci. 2021 Jul 29;8:705133. doi: 10.3389/fmolb.2021.705133. eCollection 2021. Front Mol Biosci. 2021. PMID: 34395532 Free PMC article. Review.
-
Dual role of fucosidase in cancers and its clinical potential.J Cancer. 2022 Aug 15;13(10):3121-3132. doi: 10.7150/jca.75840. eCollection 2022. J Cancer. 2022. PMID: 36046653 Free PMC article. Review.
-
Multi-slice computed tomography radiomics combined with serum alpha-L-fucosidase: a potential biomarker for precise identification of pleomorphic adenoma and Warthin tumor.Transl Cancer Res. 2024 Dec 31;13(12):6793-6806. doi: 10.21037/tcr-24-871. Epub 2024 Dec 27. Transl Cancer Res. 2024. PMID: 39816553 Free PMC article.
-
Enzymatic activity of glycosyltransferase GLT8D1 promotes human glioblastoma cell migration.iScience. 2022 Feb 2;25(2):103842. doi: 10.1016/j.isci.2022.103842. eCollection 2022 Feb 18. iScience. 2022. PMID: 35198895 Free PMC article.
References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–74. - PubMed
-
- Vajaria BN, Patel PS. Glycosylation: A hallmark of cancer? Glycoconj J. 2017;34:147–56. - PubMed
-
- Miyoshi E, Uozumi N, Sobajima T, Takamatsu S, Kamada Y. In: Mechanisms of Malignant Phenotypes. Springer: Japan; 2016. Roles of fucosyltransferases in cancer phenotypes. Glycosignals in cancer; pp. 3–16.
-
- Shah MH, Telang SD, Shah PM, Patel PS. Tissue and serum α2-3-and α2-6-linkage specific sialylation changes in oral carcinogenesis. Glycoconj J. 2008;25:279–90. - PubMed
LinkOut - more resources
Full Text Sources

