Recommendation of using systemic anti-tumor necrosis factor-alpha for the treatment of noninfectious uveitis in Taiwan
- PMID: 30294525
- PMCID: PMC6169331
- DOI: 10.4103/tjo.tjo_32_18
Recommendation of using systemic anti-tumor necrosis factor-alpha for the treatment of noninfectious uveitis in Taiwan
Abstract
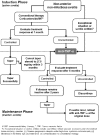
Noninfectious uveitis is a sight-threatening disease with an autoimmune or autoinflammatory basis. Systemic treatment is required if intraocular inflammation threatens a patient's vision or cannot be controlled locally and when it is associated with systemic rheumatic diseases. Corticosteroids and immunomodulatory chemotherapy are the conventional initial treatments. However, the various side effects of these therapies increase the burden on patients, not only physically but also mentally. Moreover, uncontrolled inflammation and poor visual outcomes have sometimes been recorded despite the combination of these medications or their high dosage. Antitumor necrosis factor-alpha (anti-TNF-α) and other biologic agents have been widely used to treat rheumatic diseases for >15 years. Randomized controlled clinical trials have demonstrated that anti-TNF-α can reduce and delay episodes of intraocular inflammation not only in patients with active uveitis but also in corticosteroid-dependent patients with inactive uveitis. The Taiwan Food and Drug Administration approved the use of adalimumab, an anti-TNF-α agent, for treating nonanterior noninfectious uveitis (NANIU) in 2017. This report provides a recommendation and a proposed stepladder approach for using anti-TNF-α agents to treat NANIU in Taiwan.
Keywords: Adalimumab; immunomodulatory; noninfectious; treatment; tumor necrosis factor; uveitis.
Conflict of interest statement
The authors declare that there are no conflicts of interests of this paper.
Figures
References
-
- Durrani OM, Meads CA, Murray PI. Uveitis: A potentially blinding disease. Ophthalmologica. 2004;218:223–36. - PubMed
-
- Sève P, Cacoub P, Bodaghi B, Trad S, Sellam J, Bellocq D, et al. Uveitis: Diagnostic work-up. A literature review and recommendations from an expert committee. Autoimmun Rev. 2017;16:1254–64. - PubMed