Genome Editing Reveals Idiosyncrasy of CNGA2 Ion Channel-Directed Antibody Immunoreactivity Toward Oxytocin
- PMID: 30294598
- PMCID: PMC6158348
- DOI: 10.3389/fcell.2018.00117
Genome Editing Reveals Idiosyncrasy of CNGA2 Ion Channel-Directed Antibody Immunoreactivity Toward Oxytocin
Abstract
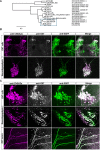
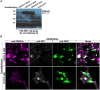
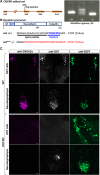
Presynaptic cGMP-gated ion (CNG) channels positively or negatively modulate neurotransmitter secretion as well as the strength of synaptic transmission. Zebrafish cGMP-gated ion channel, CNGA2a (a.k.a. CNGA5), was previously reported to be specifically enriched in synaptic terminals of zebrafish oxytocin (OXT) neurons. This conclusion was based on immunoreactivity of a monoclonal antibody (mAb) clone L55/54, which was directed against the carboxy terminal tail of the CNGA2a. To study the role of CNGA2a in oxytocin neurons function, we generated zebrafish mutants of cnga2a, cnga2b and oxt genes using clustered regularly interspaced short palindromic repeats (CRISPR)-mediated genome editing. We show that mAb L55/54 specifically recognizes CNGA2a protein when expressed in heterologous cell culture system. Surprisingly, anti-CNGA2a immunoreactivity was not eliminated following knockout of either cnga2a, cnga2b or both. However, knockout of oxt resulted in total loss of anti-CNGA2a mAb immunoreactivity despite the lack of sequence and structural similarities between OXT and CNGA2a proteins. Our results provide a noteworthy lesson of differences in antibody immunoreactivity, which could only be revealed using specific genetic tools.
Keywords: cGMP-gated ion channel; monoclonal antibody; neurohypophysis; neuropeptide; oxytocin.
Figures


Similar articles
-
A novel cyclic nucleotide-gated ion channel enriched in synaptic terminals of isotocin neurons in zebrafish brain and pituitary.Neuroscience. 2010 Jan 13;165(1):79-89. doi: 10.1016/j.neuroscience.2009.09.040. Epub 2009 Sep 22. Neuroscience. 2010. PMID: 19778592 Free PMC article.
-
Characterization of a novel cyclic nucleotide-gated channel from zebrafish brain.Biochem Biophys Res Commun. 2006 Sep 22;348(2):441-9. doi: 10.1016/j.bbrc.2006.07.074. Epub 2006 Jul 28. Biochem Biophys Res Commun. 2006. PMID: 16887101
-
Genomic editing opens new avenues for zebrafish as a model for neurodegeneration.J Neurochem. 2013 Nov;127(4):461-70. doi: 10.1111/jnc.12460. Epub 2013 Oct 21. J Neurochem. 2013. PMID: 24117801
-
Modulation of synaptic function by cGMP and cGMP-gated cation channels.Neurochem Int. 2004 Nov;45(6):875-84. doi: 10.1016/j.neuint.2004.03.018. Neurochem Int. 2004. PMID: 15312982 Review.
-
Genome editing using artificial site-specific nucleases in zebrafish.Dev Growth Differ. 2014 Jan;56(1):26-33. doi: 10.1111/dgd.12094. Epub 2013 Oct 14. Dev Growth Differ. 2014. PMID: 24117409 Review.
Cited by
-
Agouti-Related Protein 2 Is a New Player in the Teleost Stress Response System.Curr Biol. 2019 Jun 17;29(12):2009-2019.e7. doi: 10.1016/j.cub.2019.05.021. Epub 2019 Jun 6. Curr Biol. 2019. PMID: 31178320 Free PMC article.
-
Perceptual mechanisms of social affiliation in zebrafish.Sci Rep. 2020 Feb 27;10(1):3642. doi: 10.1038/s41598-020-60154-8. Sci Rep. 2020. PMID: 32107434 Free PMC article.
-
Splice-specific deficiency of the PTSD-associated gene PAC1 leads to a paradoxical age-dependent stress behavior.Sci Rep. 2020 Jun 12;10(1):9559. doi: 10.1038/s41598-020-66447-2. Sci Rep. 2020. PMID: 32533011 Free PMC article.
-
Developmental Effects of Oxytocin Neurons on Social Affiliation and Processing of Social Information.J Neurosci. 2021 Oct 20;41(42):8742-8760. doi: 10.1523/JNEUROSCI.2939-20.2021. Epub 2021 Sep 1. J Neurosci. 2021. PMID: 34470805 Free PMC article.
References
-
- Bradley J., Zhang Y., Bakin R., Lester H. A., Ronnett G. V., Zinn K. (1997). Functional expression of the heteromeric “olfactory” cyclic nucleotide-gated channel in the hippocampus: a potential effector of synaptic plasticity in brain neurons. J. Neurosci. 17 1993–2005. 10.1523/JNEUROSCI.17-06-01993.1997 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

