Two-step recruitment process optimizes retention in FLEX clinical trial
- PMID: 30294698
- PMCID: PMC6169149
- DOI: 10.1016/j.conctc.2018.09.005
Two-step recruitment process optimizes retention in FLEX clinical trial
Erratum in
-
Erratum regarding missing Declaration of Competing Interest statements in previously published articles.Contemp Clin Trials Commun. 2020 Dec 10;20:100688. doi: 10.1016/j.conctc.2020.100688. eCollection 2020 Dec. Contemp Clin Trials Commun. 2020. PMID: 33392412 Free PMC article.
Abstract
Introduction: The Flexible Lifestyle Empowering Change Study (FLEX) is a multi-site randomized controlled trial to test the efficacy of an adaptive behavioral intervention to promote self-management and improve glycemic control for adolescents with type 1 diabetes mellitus. A two-step recruitment process was used to optimize study retention by facilitating informed decision-making regarding participation.
Methods: Those who expressed interest at first contact were given more detailed study information followed by telephone calls to the adolescents and their parents to answer questions and explore potential barriers to participation before making a decision regarding study enrollment.
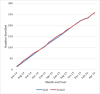
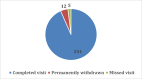
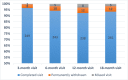
Results: Of 694 eligible adolescents who were invited to participate, 397 (57.2%) expressed interest when initially contacted (Step 1). Upon completion of the follow-up telephone calls (Step 2), 276 (39.8%) still agreed to participate; and 258 (37.2%) enrolled and completed a baseline visit with a parent/guardian. Completion rates for measurement visits remained high throughout the study, with an end-of-study retention rate of 93.4%; and only 12 (4.7%) families withdrew from the study.
Conclusion: The two-step recruitment process encourages potential participants to thoughtfully evaluate their willingness to participate, as well as their ability to make a commitment to the full completion of study requirements. When demonstrating the efficacy of a randomized controlled trial, it may be preferable to accept lower recruitment rates in order to optimize retention rates. The additional time and effort required to implement this two-step process is worthwhile. With a high retention rate, we can be more confident that the outcomes of the randomized controlled trial actually reflect the impact of the intervention.
Keywords: Adolescent; Randomized controlled trial; Recruitment; Retention; Type 1 diabetes.
Figures
References
-
- Lovato L.C., Hill K., Hertert S., Hunninghake D.B., Probstfield J.L. Recruitment for controlled clinical trials: literature summary and annotated bibliography, Control. Clin. Trials. 1997;18:328–352. - PubMed
-
- Løding R.N., Wold J.E., Skavhaug Å., Graue M. Evaluation of peer-group support and problem-solving training in the treatment of adolescents with type 1 diabetes. Eur. Diabetes Nurs. 2007;4:28–33.
-
- Christie D., Thompson R., Sawtell M., Allen E., Cairns J., Smith F., Jamieson E., Hargreaves K., Ingold A., Brooks L., Wiggins M., Oliver S., Jones R., Elbourne D., Santos A., Wong I.C.K., O'Neill S., Strange V., Hindmarsh P., Annan F., Viner R. Structured, intensive education maximising engagement, motivation and long-term change for children and young people with diabetes: a cluster randomised controlled trial with integral process and economic evaluation - the CASCADE study. Health Technol. Assess. (Rockv). 2014;18:1–202. - PMC - PubMed
-
- Marcellus L. Are we missing anything? Pursuing research on attrition. Can. J. Nurs. Res. 2004;36:82–98. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources