Towards take-all control: a C-21β oxidase required for acylation of triterpene defence compounds in oat
- PMID: 30294977
- PMCID: PMC6446040
- DOI: 10.1111/nph.15456
Towards take-all control: a C-21β oxidase required for acylation of triterpene defence compounds in oat
Abstract
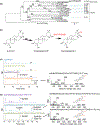
Oats produce avenacins, antifungal triterpenes that are synthesized in the roots and provide protection against take-all and other soilborne diseases. Avenacins are acylated at the carbon-21 position of the triterpene scaffold, a modification critical for antifungal activity. We have previously characterized several steps in the avenacin pathway, including those required for acylation. However, transfer of the acyl group to the scaffold requires the C-21β position to be oxidized first, by an as yet uncharacterized enzyme. We mined oat transcriptome data to identify candidate cytochrome P450 enzymes that may catalyse C-21β oxidation. Candidates were screened for activity by transient expression in Nicotiana benthamiana. We identified a cytochrome P450 enzyme AsCYP72A475 as a triterpene C-21β hydroxylase, and showed that expression of this enzyme together with early pathway steps yields C-21β oxidized avenacin intermediates. We further demonstrate that AsCYP72A475 is synonymous with Sad6, a previously uncharacterized locus required for avenacin biosynthesis. sad6 mutants are compromised in avenacin acylation and have enhanced disease susceptibility. The discovery of AsCYP72A475 represents an important advance in the understanding of triterpene biosynthesis and paves the way for engineering the avenacin pathway into wheat and other cereals for control of take-all and other diseases.
Keywords: Avena strigosa; avenacins; cytochromes P450; disease resistance; metabolic engineering; natural products; triterpenes.
© 2018 The Authors. New Phytologist © 2018 New Phytologist Trust.
Figures




References
-
- Asher MJC, Shipton PJ. 1981. Biology and control of take-all. London, UK: Academic Press.
-
- Biazzi E, Carelli M, Tava A, Abbruscato P, Losini I, Avato P, Scotti C, Calderini O. 2015. CYP72A67 catalyzes a key oxidative step in Medicago truncatula hemolytic saponin biosynthesis. Molecular Plant 8: 1493–1506. - PubMed
-
- Chan PK. 2007. Acylation with diangeloyl groups at C21–22 positions in triterpenoid saponins is essential for cytotoxicity towards tumor cells. Biochemical Pharmacology 73: 341–350. - PubMed
-
- Chiba Y, Green PJ. 2009. mRNA degradation machinery in plants. Journal of Plant Biology 52: 114–124.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
- BBS/E/J/000PR9790/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/L014130/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- joint Engineering and Physical Sciences Research Council/International
- BB/K005952/1/BBSRC/International
- 2016QNRC001/Young Elite Scientists Sponsorship Program/International
- John Innes Foundation/International
- U01 GM110699/GM/NIGMS NIH HHS/United States
- BB/H009582/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- U101GM110699/National Institutes of Health Genome to Natural Products Network award/International
- BB/E009912/1/BBSRC/International
- BBS/E/J/00000614/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/L014130/1/OpenPlant Synthetic Biology Research Centre/International
- BB/H009582/1/BBSRC/International
- CAST/International
- China Scholarship Council/International
LinkOut - more resources
Full Text Sources
Other Literature Sources

