Replication Study: Intestinal inflammation targets cancer-inducing activity of the microbiota
- PMID: 30295289
- PMCID: PMC6175580
- DOI: 10.7554/eLife.34364
Replication Study: Intestinal inflammation targets cancer-inducing activity of the microbiota
Abstract
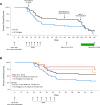
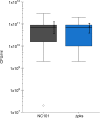
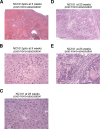
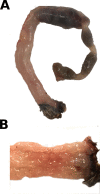
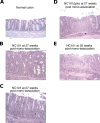
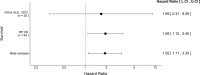
As part of the Reproducibility Project: Cancer Biology we published a Registered Report (Eaton et al., 2015) that described how we intended to replicate selected experiments from the paper "Intestinal Inflammation Targets Cancer-Inducing Activity of the Microbiota" (Arthur et al., 2012). Here we report the results. We observed no impact on bacterial growth or colonization capacity when the polyketide synthase (pks) genotoxic island was deleted from E. coli NC101, similar to the original study (Supplementary Figure 7; Arthur et al., 2012). However, for the experiment that compared inflammation, invasion, and neoplasia in azoxymethane (AOM)-treated interleukin-10-deficient mice mono-associated with NC101 or NC101[Formula: see text] pks the experimental timing of the replication attempt was longer than that of the original study. This difference was because in the original study the methodology was not clearly stated and likely led to the increased mortality and severity of inflammation observed in this replication attempt. Additionally, early death occurred during AOM treatment with higher mortality observed in NC101[Formula: see text] pks mono-associated mice compared to NC101, which was in the same direction, but more severe than the original study (Suppleme1ntal Figure 10; Arthur et al., 2012). A meta-analysis suggests that mice mono-associated with NC101[Formula: see text] pks have higher mortality compared to NC101. While these data were unable to address whether, under the conditions of the original study, NC101 and NC101[Formula: see text] pks differ in inflammation, invasion, and neoplasia this replication attempt demonstrates that clear description of experimental methods is essential to ensure accurate reproduction of experimental studies.
Keywords: E. coli; Intestinal Inflammation; Reproducibility Project: Cancer Biology; cancer biology; metascience; microbiota; mouse; replication; reproducibility.
© 2018, Eaton et al.
Conflict of interest statement
KE Germ-Free and Gnotobiotic Mouse Facilities, University of Michigan Medical School was a Science Exchange associated lab. AP, ES No competing interests declared, EI, RT, NP: Employed by and hold shares in Science Exchange Inc.
Figures





Comment in
-
Five keys to writing a reproducible lab protocol.Nature. 2021 Sep;597(7875):293-294. doi: 10.1038/d41586-021-02428-3. Nature. 2021. PMID: 34489578 No abstract available.
References
-
- Andrews S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Cambridge, UK: Bioinformatics Group at the Babraham Institute; 2016.
-
- Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

