Angiopoietin-2 exacerbates cardiac hypoxia and inflammation after myocardial infarction
- PMID: 30295643
- PMCID: PMC6205384
- DOI: 10.1172/JCI99659
Angiopoietin-2 exacerbates cardiac hypoxia and inflammation after myocardial infarction
Abstract
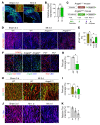
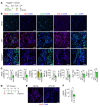
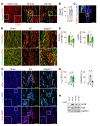
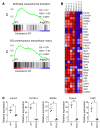
Emerging evidence indicates that angiopoietin-2 (Angpt2), a well-recognized vascular destabilizing factor, is a biomarker of poor outcome in ischemic heart disease. However, its precise role in postischemic cardiovascular remodeling is poorly understood. Here, we show that Angpt2 plays multifaceted roles in the exacerbation of cardiac hypoxia and inflammation after myocardial ischemia. Angpt2 was highly expressed in endothelial cells at the infarct border zone after myocardial infarction (MI) or ischemia/reperfusion injury in mice. In the acute phase of MI, endothelial-derived Angpt2 antagonized Angpt1/Tie2 signaling, which was greatly involved in pericyte detachment, vascular leakage, increased adhesion molecular expression, degradation of the glycocalyx and extracellular matrix, and enhanced neutrophil infiltration and hypoxia in the infarct border area. In the chronic remodeling phase after MI, endothelial- and macrophage-derived Angpt2 continuously promoted abnormal vascular remodeling and proinflammatory macrophage polarization through integrin α5β1 signaling, worsening cardiac hypoxia and inflammation. Accordingly, inhibition of Angpt2 either by gene deletion or using an anti-Angpt2 blocking antibody substantially alleviated these pathological findings and ameliorated postischemic cardiovascular remodeling. Blockade of Angpt2 thus has potential as a therapeutic option for ischemic heart failure.
Keywords: Cardiology; Cardiovascular disease; Pericytes; Vascular Biology; endothelial cells.
Conflict of interest statement
Figures








References
-
- World Health Organization. The top 10 causes of death. http://www.who.int/mediacentre/factsheets/fs310/en/ WHO website. Published May 24, 2018. Accessed September 25, 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

