Biomarkers Are Associated With Clinical and Endoscopic Outcomes With Vedolizumab Treatment in Ulcerative Colitis
- PMID: 30295781
- PMCID: PMC6327228
- DOI: 10.1093/ibd/izy307
Biomarkers Are Associated With Clinical and Endoscopic Outcomes With Vedolizumab Treatment in Ulcerative Colitis
Abstract
Background: Vedolizumab inhibits α4β7-mediated lymphocyte trafficking and is effective in ulcerative colitis (UC). This study evaluated drug and biomarker concentrations and patient outcomes during vedolizumab treatment in UC.
Methods: Prospectively scored maintenance clinical (26.5 weeks; interquartile range [IQR], 16.3-37.0 weeks) and endoscopic (23.5 weeks; IQR, 16.8-35.6 weeks) outcomes were compared with serum vedolizumab concentrations, antivedolizumab antibodies, and serum biomarkers at baseline and weeks 2, 6, 14, and 26. A linear mixed-effects model compared biomarker trajectories over time between clinical and endoscopic remitters and nonremitters.
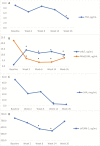
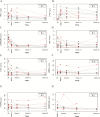
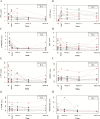
Results: Thirty-two patients were included. Soluble (s)-tumor necrosis factor (TNF)-α, s-α4β7, s-mucosal addressin cell adhesion molecule (s-MAdCAM-1), and s-amyloid A (s-AA) significantly changed with treatment. A linear mixed-effects model demonstrated that s-α4β7 (P = 0.044) increased and s-MAdCAM-1 (P = 0.006) and s-vascular cell adhesion molecule-1 (s-VCAM-1, P = 0.001) decreased more rapidly in patients achieving clinical remission in maintenance. S-MAdCAM-1 (P = 0.005), s-intracellular adhesion molecule-1 (ICAM-1; P = 0.014), s-VCAM-1 (P < 0.001), and s-TNF (P = 0.052) decreased more rapidly in endoscopic remitters. In clinical remitters, higher week 14 (20.3 ng/mL vs 6.0 ng/mL; P = 0.013) and week 26 (14.1 ng/mL vs 8.6 ng/mL; P = 0.05) s-α4β7 were observed. In endoscopic remitters, week 2 (6.7 pg/mL vs 17.8 pg/mL; P = 0.038) and week 6 (3.9 pg/mL vs 15.6 pg/mL; P = 0.005) s-TNF and week 14 s-VCAM (589.1 ng/mL vs 746.0 ng/mL; P = 0.05) were lower.
Conclusion: Serum biomarkers were associated with outcomes in vedolizumab-treated UC patients. s-α4β7 increased, whereas s-MAdCAM-1, s-VCAM-1, s-ICAM-1, and s-TNF decreased more rapidly in remitters. At individual time points, induction s-TNF and maintenance s-VCAM-1 concentrations were lower, whereas maintenance s-α4β7 concentrations were higher in remitters.
Figures
References
-
- Soler D, Chapman T, Yang LL, et al. . The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330:864–875. - PubMed
-
- Feagan BG, Rutgeerts P, Sands BE, et al. ; GEMINI 1 Study Group Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. - PubMed
-
- Sandborn WJ, Feagan BG, Rutgeerts P, et al. ; GEMINI 2 Study Group Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–721. - PubMed
-
- Sands BE, Feagan BG, Rutgeerts P, et al. . Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618–627.e3. - PubMed
-
- Mosli MH, MacDonald JK, Bickston SJ, et al. . Vedolizumab for induction and maintenance of remission in ulcerative colitis: a Cochrane systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21:1151–1159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

