The Ability of a Cytomegalovirus ELISPOT Assay to Predict Outcome of Low-Level CMV Reactivation in Hematopoietic Cell Transplant Recipients
- PMID: 30295846
- PMCID: PMC6386808
- DOI: 10.1093/infdis/jiy592
The Ability of a Cytomegalovirus ELISPOT Assay to Predict Outcome of Low-Level CMV Reactivation in Hematopoietic Cell Transplant Recipients
Abstract
Background: Cytomegalovirus (CMV) infections in hematopoietic cell transplant (HCT) recipients cause substantial morbidity and mortality. CMV cell-mediated immunity (CMV-CMI) can be determined by levels of interferon gamma (IFN-γ) production using an enzyme-linked immunospot (ELISPOT) CMV assay (T-SPOT.CMV assay). In this study, we evaluated the ability of this assay to predict the outcome of low-level CMV reactivation in HCT recipients.
Methods: We followed 55 HCT recipients with low-level CMV reactivation up to 8 weeks from enrollment. Progression to clinically significant CMV infection (CS-CMVi) was defined as a CMV load >1000 IU/mL or > 500 IU/mL in patients receiving matched related/autologous or matched unrelated transplants, respectively, and initiation of antiviral treatment.
Results: Progression to CS-CMVi occurred in 31 (56%) of the HCT recipients. Spot counts of CMV-specific pp65 and IE1 antigens were significantly lower in patients who had CS-CMVi than in patients who did not. On multivariate analysis, the ELISPOT CMV responses and steroids use were the only predictors of progression to CS-CMVi.
Conclusions: A strong association between low CMV-CMI and progression to CS-CMVi was observed in HCT recipients. The implementation of serial monitoring of CMV-CMI may identify patients at risk of progression to CS-CMVi that require antiviral therapy.
Keywords: ELISPOT assay; cell-mediated immunity; cytomegalovirus; hematopoietic stem cell transplant; low-level CMV reactivation.
© The Author(s) 2018. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

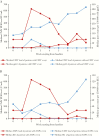

Comment in
-
Reply to Giménez et al.J Infect Dis. 2019 Apr 16;219(9):1512-1513. doi: 10.1093/infdis/jiy687. J Infect Dis. 2019. PMID: 30496440 Free PMC article. No abstract available.
-
Failure of Cytomegalovirus-Specific CD8+ T Cell Levels at Viral DNAemia Onset to Predict the Eventual Need for Preemptive Antiviral Therapy in Allogeneic Hematopoietic Stem Cell Transplant Recipients.J Infect Dis. 2019 Apr 16;219(9):1510-1512. doi: 10.1093/infdis/jiy746. J Infect Dis. 2019. PMID: 30597050 No abstract available.
References
-
- Boeckh M, Nichols WG. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood 2004; 103:2003–8. - PubMed
-
- Gandhi MK, Khanna R. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect Dis 2004; 4:725–38. - PubMed
-
- Ariza-Heredia EJ, Nesher L, Chemaly RF. Cytomegalovirus diseases after hematopoietic stem cell transplantation: a mini-review. Cancer Lett 2014; 342:1–8. - PubMed

