A SOC1-like gene MtSOC1a promotes flowering and primary stem elongation in Medicago
- PMID: 30295903
- PMCID: PMC6137972
- DOI: 10.1093/jxb/ery284
A SOC1-like gene MtSOC1a promotes flowering and primary stem elongation in Medicago
Abstract
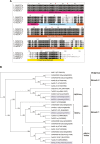
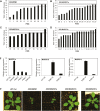
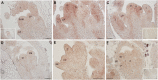
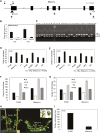
Medicago flowering, like that of Arabidopsis, is promoted by vernalization and long days, but alternative mechanisms are predicted because Medicago lacks the key regulators CO and FLC. Three Medicago SOC1-like genes, including MtSOC1a, were previously implicated in flowering control, but no legume soc1 mutants with altered flowering were reported. Here, reverse transciption-quantitative PCR (RT-qPCR) indicated that the timing and magnitude of MtSOC1a expression was regulated by the flowering promoter FTa1, while in situ hybridization indicated that MtSOC1a expression increased in the shoot apical meristem during the floral transition. A Mtsoc1a mutant showed delayed flowering and short primary stems. Overexpression of MtSOC1a partially rescued the flowering of Mtsoc1a, but caused a dramatic increase in primary stem height, well before the transition to flowering. Internode cell length correlated with stem height, indicating that MtSOC1a promotes cell elongation in the primary stem. However, application of gibberellin (GA3) caused stem elongation in both the wild type and Mtsoc1a, indicating that the mutant was not defective in gibberellin responsiveness. These results indicate that MtSOC1a may function as a floral integrator gene and promotes primary stem elongation. Overall, this study suggests that apart from some conservation with the Arabidopsis flowering network, MtSOC1a has a novel role in regulating aspects of shoot architecture.
Figures


Similar articles
-
Medicago PHYA promotes flowering, primary stem elongation and expression of flowering time genes in long days.BMC Plant Biol. 2020 Jul 11;20(1):329. doi: 10.1186/s12870-020-02540-y. BMC Plant Biol. 2020. PMID: 32652925 Free PMC article.
-
Gene-edited Mtsoc1 triple mutant Medicago plants do not flower.Front Plant Sci. 2024 Feb 26;15:1357924. doi: 10.3389/fpls.2024.1357924. eCollection 2024. Front Plant Sci. 2024. PMID: 38469328 Free PMC article.
-
Medicago truncatula SOC1 Genes Are Up-regulated by Environmental Cues That Promote Flowering.Front Plant Sci. 2018 Apr 27;9:496. doi: 10.3389/fpls.2018.00496. eCollection 2018. Front Plant Sci. 2018. PMID: 29755488 Free PMC article.
-
Regulation and function of SOC1, a flowering pathway integrator.J Exp Bot. 2010 May;61(9):2247-54. doi: 10.1093/jxb/erq098. Epub 2010 Apr 22. J Exp Bot. 2010. PMID: 20413527 Review.
-
Make hay when the sun shines: the role of MADS-box genes in temperature-dependant seasonal flowering responses.Plant Sci. 2011 Mar;180(3):447-53. doi: 10.1016/j.plantsci.2010.12.001. Epub 2010 Dec 14. Plant Sci. 2011. PMID: 21421391 Review.
Cited by
-
Medicago PHYA promotes flowering, primary stem elongation and expression of flowering time genes in long days.BMC Plant Biol. 2020 Jul 11;20(1):329. doi: 10.1186/s12870-020-02540-y. BMC Plant Biol. 2020. PMID: 32652925 Free PMC article.
-
Genomic and Transcriptomic Insights into the Evolution and Divergence of MIKC-Type MADS-Box Genes in Carica papaya.Int J Mol Sci. 2023 Sep 13;24(18):14039. doi: 10.3390/ijms241814039. Int J Mol Sci. 2023. PMID: 37762345 Free PMC article.
-
Height to first pod: A review of genetic and breeding approaches to improve combine harvesting in legume crops.Front Plant Sci. 2022 Sep 16;13:948099. doi: 10.3389/fpls.2022.948099. eCollection 2022. Front Plant Sci. 2022. PMID: 36186054 Free PMC article. Review.
-
Differential roles of Cassia tora 1-deoxy-D-xylulose-5-phosphate synthase and 1-deoxy-D-xylulose-5-phosphate reductoisomerase in trade-off between plant growth and drought tolerance.Front Plant Sci. 2023 Oct 20;14:1270396. doi: 10.3389/fpls.2023.1270396. eCollection 2023. Front Plant Sci. 2023. PMID: 37929171 Free PMC article.
-
Longer Internode with Same Cell Length: LcSOC1-b2 Gene Involved in Height to First Pod but Not Flowering in Lentil (Lens culinaris Medik.).Plants (Basel). 2025 Apr 8;14(8):1157. doi: 10.3390/plants14081157. Plants (Basel). 2025. PMID: 40284045 Free PMC article.

