Molecular Targeted NIR-II Probe for Image-Guided Brain Tumor Surgery
- PMID: 30296054
- PMCID: PMC6363276
- DOI: 10.1021/acs.bioconjchem.8b00669
Molecular Targeted NIR-II Probe for Image-Guided Brain Tumor Surgery
Abstract
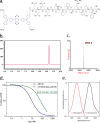
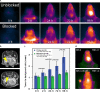
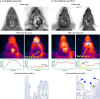
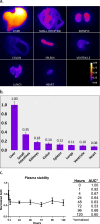
Optical imaging strategies for improving delineation of glioblastoma (GBM) is highly desired for guiding surgeons to distinguish cancerous tissue from healthy and precious brain tissue. Fluorescence imaging (FLI) in the second near-infrared window (NIR-II) outperforms traditional NIR-I imaging with better tissue penetration, higher spatial and temporal resolution, and less auto fluorescence and scattering. Because of high expression in GBM and many other tumors, urokinase Plasminogen Activator Receptor (uPAR) is an attractive and well proven target for FLI. Herein we aim to combine the benefit of a NIR-II fluorophore with a high affinity uPAR targeting small peptide. A targeted NIR-II fluorescent probe was developed by conjugating an in-house synthesized NIR-II fluorophore, CH1055, and a uPAR targeting peptide, AE105. To characterize the in vivo distribution and targeting properties, a dynamic imaging was performed in orthotopic GBM bearing nude mice ( n = 8). Additionally, fluorescence guided surgery of orthotopic GBM was performed in living animals. CH1055-4Glu-AE105 was easily synthesized with >75% yield and >98% HPLC evaluated purity. The retention time of the probe on analytical HPLC was 15.9 min and the product was verified by mass spectrometry. Dynamic imaging demonstrated that the uPAR targeting probe visualized orthotopic GBM through the intact skull with a tumor-to-background ratio (TBR) of 2.7 peaking at 96 h. Further, the orthotopic GBM was successfully resected in small animals guided by the NIR-II FLI. By using a small uPAR targeting NIR-II probe, FLI allows us to specifically image and detect GBM. A real-time imaging setup further renders FLI guided tumor resection, and the probe developed in this work is a promising candidate for clinical translation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
uPAR-targeted optical near-infrared (NIR) fluorescence imaging and PET for image-guided surgery in head and neck cancer: proof-of-concept in orthotopic xenograft model.Oncotarget. 2017 Feb 28;8(9):15407-15419. doi: 10.18632/oncotarget.14282. Oncotarget. 2017. PMID: 28039488 Free PMC article.
-
IRDye800CW labeled uPAR-targeting peptide for fluorescence-guided glioblastoma surgery: Preclinical studies in orthotopic xenografts.Theranostics. 2021 May 21;11(15):7159-7174. doi: 10.7150/thno.49787. eCollection 2021. Theranostics. 2021. PMID: 34158842 Free PMC article.
-
PET/NIR-II fluorescence imaging and image-guided surgery of glioblastoma using a folate receptor α-targeted dual-modal nanoprobe.Eur J Nucl Med Mol Imaging. 2022 Nov;49(13):4325-4337. doi: 10.1007/s00259-022-05890-x. Epub 2022 Jul 15. Eur J Nucl Med Mol Imaging. 2022. PMID: 35838757
-
Inorganic and hybrid nanomaterials for NIR-II fluorescence imaging-guided therapy of Glioblastoma and perspectives.Theranostics. 2025 Apr 21;15(12):5616-5665. doi: 10.7150/thno.112204. eCollection 2025. Theranostics. 2025. PMID: 40365286 Free PMC article. Review.
-
Surgical Navigation for Malignancies Guided by Near-Infrared-II Fluorescence Imaging.Small Methods. 2021 Mar;5(3):e2001066. doi: 10.1002/smtd.202001066. Epub 2021 Jan 18. Small Methods. 2021. PMID: 34927825 Review.
Cited by
-
Progress and Viewpoints of Multifunctional Composite Nanomaterials for Glioblastoma Theranostics.Pharmaceutics. 2022 Feb 21;14(2):456. doi: 10.3390/pharmaceutics14020456. Pharmaceutics. 2022. PMID: 35214188 Free PMC article. Review.
-
Nanoprobe-Based Near-Infrared II Optical Imaging for Guiding Precision Glioma Therapy.Int J Nanomedicine. 2025 Jun 27;20:8433-8449. doi: 10.2147/IJN.S523676. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40599397 Free PMC article. Review.
-
Evolution and Medical Significance of LU Domain-Containing Proteins.Int J Mol Sci. 2019 Jun 5;20(11):2760. doi: 10.3390/ijms20112760. Int J Mol Sci. 2019. PMID: 31195646 Free PMC article. Review.
-
Unveiling the Crucial Roles of O2•- and ATP in Hepatic Ischemia-Reperfusion Injury Using Dual-Color/Reversible Fluorescence Imaging.J Am Chem Soc. 2023 Sep 13;145(36):19662-19675. doi: 10.1021/jacs.3c04303. Epub 2023 Sep 1. J Am Chem Soc. 2023. PMID: 37655757 Free PMC article.
-
Application of Nanoparticles in the Diagnosis and Treatment of Colorectal Cancer.Anticancer Agents Med Chem. 2024;24(18):1305-1326. doi: 10.2174/0118715206323900240807110122. Anticancer Agents Med Chem. 2024. PMID: 39129164 Free PMC article. Review.
References
-
- Stummer W.; Reulen H. J.; Meinel T.; Pichlmeier U.; Schumacher W.; Tonn J. C.; Rohde V.; Oppel F.; Turowski B.; Woiciechowsky C.; et al. (2008) Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery 62, 564–76. discussion 564–76.10.1227/01.neu.0000317304.31579.17. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

