Interactive reservoir computing for chunking information streams
- PMID: 30296262
- PMCID: PMC6193738
- DOI: 10.1371/journal.pcbi.1006400
Interactive reservoir computing for chunking information streams
Abstract
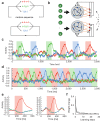
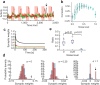
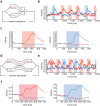
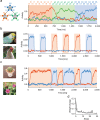
Chunking is the process by which frequently repeated segments of temporal inputs are concatenated into single units that are easy to process. Such a process is fundamental to time-series analysis in biological and artificial information processing systems. The brain efficiently acquires chunks from various information streams in an unsupervised manner; however, the underlying mechanisms of this process remain elusive. A widely-adopted statistical method for chunking consists of predicting frequently repeated contiguous elements in an input sequence based on unequal transition probabilities over sequence elements. However, recent experimental findings suggest that the brain is unlikely to adopt this method, as human subjects can chunk sequences with uniform transition probabilities. In this study, we propose a novel conceptual framework to overcome this limitation. In this process, neural networks learn to predict dynamical response patterns to sequence input rather than to directly learn transition patterns. Using a mutually supervising pair of reservoir computing modules, we demonstrate how this mechanism works in chunking sequences of letters or visual images with variable regularity and complexity. In addition, we demonstrate that background noise plays a crucial role in correctly learning chunks in this model. In particular, the model can successfully chunk sequences that conventional statistical approaches fail to chunk due to uniform transition probabilities. In addition, the neural responses of the model exhibit an interesting similarity to those of the basal ganglia observed after motor habit formation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

