Light-induced generation and toxicity of docosahexaenoate-derived oxidation products in retinal pigmented epithelial cells
- PMID: 30296412
- PMCID: PMC6443467
- DOI: 10.1016/j.exer.2018.09.012
Light-induced generation and toxicity of docosahexaenoate-derived oxidation products in retinal pigmented epithelial cells
Abstract
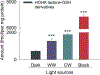
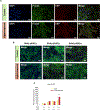
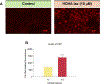
Oxidative cleavage of docosahexaenoate (DHA) in retinal pigmented epithelial (RPE) cells produces 4-hydroxy-7-oxohept-5-enoic acid (HOHA) esters of 2-lysophosphatidylcholine (PC). HOHA-PC spontaneously releases a membrane-permeant HOHA lactone that modifies primary amino groups of proteins and ethanolamine phospholipids to produce 2-(ω-carboxyethyl)pyrrole (CEP) derivatives. CEPs have significant pathological relevance to age-related macular degeneration (AMD) including activation of CEP-specific T-cells leading to inflammatory M1 polarization of macrophages in the retina involved in "dry AMD" and TLR2-dependent induction of angiogenesis that characterizes "wet AMD". RPE cells accumulate DHA from shed rod photoreceptor outer segments through phagocytosis and from plasma lipoproteins secreted by the liver through active uptake from the choriocapillaris. As a cell model of light-induced oxidative damage of DHA phospholipids in RPE cells, ARPE-19 cells were supplemented with DHA, with or without the lipofuscin fluorophore A2E. In this model, light exposure, in the absence of A2E, promoted the generation HOHA lactone-glutathione (GSH) adducts, depletion of intracellular GSH and a competing generation of CEPs. While DHA-rich RPE cells exhibit an inherent proclivity toward light-induced oxidative damage, photosensitization by A2E nearly doubled the amount of lipid oxidation and expanded the spectral range of photosensitivity to longer wavelengths. Exposure of ARPE-19 cells to 1 μM HOHA lactone for 24 h induced massive (50%) loss of lysosomal membrane integrity and caused loss of mitochondrial membrane potential. Using senescence-associated β-galactosidase (SA β-gal) staining that detects lysosomal β-galactosidase, we determined that exposure to HOHA lactone induces senescence in ARPE-19 cells. The present study shows that products of light-induced oxidative damage of DHA phospholipids in the absence of A2E can lead to RPE cell dysfunction. Therefore, their toxicity may be especially important in the early stages of AMD before RPE cells accumulate lipofuscin fluorophores.
Keywords: A2E; Age-related macular degeneration; Carboxyethylpyrrole; Lipid oxidation; Lysosome; Mitochondrial membrane potential.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures
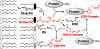



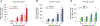
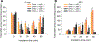


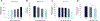




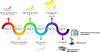
References
-
- Alary J, Fernandez Y, Debrauwer L, Perdu E, Gueraud F, 2003. Identification of intermediate pathways of 4-hydroxynonenal metabolism in the rat. Chem Res Toxicol 16, 320–327. - PubMed
-
- Anoopkumar-Dukie S, Carey JB, Conere T, O’Sullivan E, van Pelt FN, Allshire A, 2005. Resazurin assay of radiation response in cultured cells. Br J Radiol 78, 945–947. - PubMed
-
- Artigas JM, Felipe A, Navea A, Fandino A, Artigas C, 2012. Spectral transmission of the human crystalline lens in adult and elderly persons: color and total transmission of visible light. Invest Ophthalmol Vis Sci 53, 4076–4084. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

