Why did IL-23p19 inhibition fail in AS: a tale of tissues, trials or translation?
- PMID: 30297330
- PMCID: PMC6691857
- DOI: 10.1136/annrheumdis-2018-213654
Why did IL-23p19 inhibition fail in AS: a tale of tissues, trials or translation?
Keywords: DMARDs (biologic); cytokines; spondyloarthritis.
Conflict of interest statement
Competing interests: SS has received honoraria or research funding to his University from Novartis, Celgene, Janssen, Pfizer, AbbVie, UCB and Boehringer Ingelheim. NM has received honoraria or research funding to his University from Novartis and Stryker. IBM has received honoraria or research funding to his University from Novartis, Celgene, Janssen, Pfizer, AbbVie, UCB and Boehringer Ingelheim.
Figures
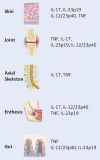
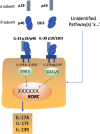
Comment on
-
Comparative genomic profiling of synovium versus skin lesions in psoriatic arthritis.Arthritis Rheumatol. 2015 Apr;67(4):934-44. doi: 10.1002/art.38995. Arthritis Rheumatol. 2015. PMID: 25512250 Free PMC article.
References
-
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol 2017;76:405–17. 10.1016/j.jaad.2016.11.041 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

