Reconfigured Cyanogenic Glucoside Biosynthesis in Eucalyptus cladocalyx Involves a Cytochrome P450 CYP706C55
- PMID: 30297456
- PMCID: PMC6236593
- DOI: 10.1104/pp.18.00998
Reconfigured Cyanogenic Glucoside Biosynthesis in Eucalyptus cladocalyx Involves a Cytochrome P450 CYP706C55
Abstract
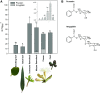
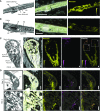
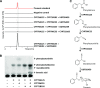
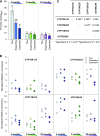
Cyanogenic glucosides are a class of specialized metabolites widespread in the plant kingdom. Cyanogenic glucosides are α-hydroxynitriles, and their hydrolysis releases toxic hydrogen cyanide, providing an effective chemical defense against herbivores. Eucalyptus cladocalyx is a cyanogenic tree, allocating up to 20% of leaf nitrogen to the biosynthesis of the cyanogenic monoglucoside, prunasin. Here, mass spectrometry analyses of E. cladocalyx tissues revealed spatial and ontogenetic variations in prunasin content, as well as the presence of the cyanogenic diglucoside amygdalin in flower buds and flowers. The identification and biochemical characterization of the prunasin biosynthetic enzymes revealed a unique enzyme configuration for prunasin production in E. cladocalyx This result indicates that a multifunctional cytochrome P450 (CYP), CYP79A125, catalyzes the initial conversion of l-phenylalanine into its corresponding aldoxime, phenylacetaldoxime; a function consistent with other members of the CYP79 family. In contrast to the single multifunctional CYP known from other plant species, the conversion of phenylacetaldoxime to the α-hydroxynitrile, mandelonitrile, is catalyzed by two distinct CYPs. CYP706C55 catalyzes the dehydration of phenylacetaldoxime, an unusual CYP reaction. The resulting phenylacetonitrile is subsequently hydroxylatedby CYP71B103 to form mandelonitrile. The final glucosylation step to yield prunasin is catalyzed by a UDP-glucosyltransferase, UGT85A59. Members of the CYP706 family have not been reported previously to participate in the biosynthesis of cyanogenic glucosides, and the pathway structure in E. cladocalyx represents an example of convergent evolution in the biosynthesis of cyanogenic glucosides in plants.
© 2018 American Society of Plant Biologists. All rights reserved.
Figures


Comment in
-
Setting and Diffusing the Cyanide Bomb in Plant Defense.Plant Physiol. 2018 Nov;178(3):956-957. doi: 10.1104/pp.18.01214. Plant Physiol. 2018. PMID: 30425157 Free PMC article. No abstract available.
References
-
- Andersen MD, Busk PK, Svendsen I, Møller BL (2000) Cytochromes P-450 from cassava (Manihot esculenta Crantz) catalyzing the first steps in the biosynthesis of the cyanogenic glucosides linamarin and lotaustralin: cloning, functional expression in Pichia pastoris, and substrate specificity of the isolated recombinant enzymes. J Biol Chem 275: 1966–1975 - PubMed
-
- Bak S, Kahn RA, Nielsen HL, Møller BL, Halkier BA (1998) Cloning of three A-type cytochromes P450, CYP71E1, CYP98, and CYP99 from Sorghum bicolor (L.) Moench by a PCR approach and identification by expression in Escherichia coli of CYP71E1 as a multifunctional cytochrome P450 in the biosynthesis of the cyanogenic glucoside dhurrin. Plant Mol Biol 36: 393–405 - PubMed
-
- Bjarnholt N, Li B, D’Alvise J, Janfelt C (2014) Mass spectrometry imaging of plant metabolites: principles and possibilities. Nat Prod Rep 31: 818–837 - PubMed
-
- Bjarnholt N, Neilson EHJ, Crocoll C, Jørgensen K, Motawia MS, Olsen CE, Dixon DP, Edwards R, Møller BL (2018) Glutathione transferases catalyze recycling of auto-toxic cyanogenic glucosides in sorghum. Plant J 94: 1109–1125 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

