Integration of gene expression and brain-wide connectivity reveals the multiscale organization of mouse hippocampal networks
- PMID: 30297807
- PMCID: PMC6398347
- DOI: 10.1038/s41593-018-0241-y
Integration of gene expression and brain-wide connectivity reveals the multiscale organization of mouse hippocampal networks
Abstract
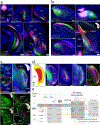
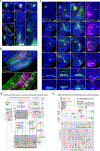
Understanding the organization of the hippocampus is fundamental to understanding brain function related to learning, memory, emotions, and diseases such as Alzheimer's disease. Physiological studies in humans and rodents have suggested that there is both structural and functional heterogeneity along the longitudinal axis of the hippocampus. However, the recent discovery of discrete gene expression domains in the mouse hippocampus has provided the opportunity to re-evaluate hippocampal connectivity. To integrate mouse hippocampal gene expression and connectivity, we mapped the distribution of distinct gene expression patterns in mouse hippocampus and subiculum to create the Hippocampus Gene Expression Atlas (HGEA). Notably, previously unknown subiculum gene expression patterns revealed a hidden laminar organization. Guided by the HGEA, we constructed the most detailed hippocampal connectome available using Mouse Connectome Project ( http://www.mouseconnectome.org ) tract tracing data. Our results define the hippocampus' multiscale network organization and elucidate each subnetwork's unique brain-wide connectivity patterns.
Conflict of interest statement
Competing Financial Interests
The authors declare no competing financial interests.
Figures






References
-
- Moser MB & Moser EI Functional differentiation in the hippocampus. Hippocampus 8, 608–619 (1998). - PubMed
-
- Poppenk J, Evensmoen HR, Moscovitch M & Nadel L Long-axis specialization of the human hippocampus. Trends in cognitive sciences 17, 230–240 (2013). - PubMed
-
- Risold P & Swanson L Structural evidence for functional domains in the rat hippocampus. Science 272, 1484 (1996). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

