High-efficiency optogenetic silencing with soma-targeted anion-conducting channelrhodopsins
- PMID: 30297821
- PMCID: PMC6175909
- DOI: 10.1038/s41467-018-06511-8
High-efficiency optogenetic silencing with soma-targeted anion-conducting channelrhodopsins
Abstract
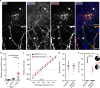
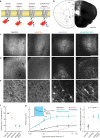
Optogenetic silencing allows time-resolved functional interrogation of defined neuronal populations. However, the limitations of inhibitory optogenetic tools impose stringent constraints on experimental paradigms. The high light power requirement of light-driven ion pumps and their effects on intracellular ion homeostasis pose unique challenges, particularly in experiments that demand inhibition of a widespread neuronal population in vivo. Guillardia theta anion-conducting channelrhodopsins (GtACRs) are promising in this regard, due to their high single-channel conductance and favorable photon-ion stoichiometry. However, GtACRs show poor membrane targeting in mammalian cells, and the activity of such channels can cause transient excitation in the axon due to an excitatory chloride reversal potential in this compartment. Here, we address these problems by enhancing membrane targeting and subcellular compartmentalization of GtACRs. The resulting soma-targeted GtACRs show improved photocurrents, reduced axonal excitation and high light sensitivity, allowing highly efficient inhibition of neuronal activity in the mammalian brain.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

