Rifamycin congeners kanglemycins are active against rifampicin-resistant bacteria via a distinct mechanism
- PMID: 30297823
- PMCID: PMC6175910
- DOI: 10.1038/s41467-018-06587-2
Rifamycin congeners kanglemycins are active against rifampicin-resistant bacteria via a distinct mechanism
Abstract
Rifamycin antibiotics (Rifs) target bacterial RNA polymerases (RNAPs) and are widely used to treat infections including tuberculosis. The utility of these compounds is threatened by the increasing incidence of resistance (RifR). As resistance mechanisms found in clinical settings may also occur in natural environments, here we postulated that bacteria could have evolved to produce rifamycin congeners active against clinically relevant resistance phenotypes. We survey soil metagenomes and identify a tailoring enzyme-rich family of gene clusters encoding biosynthesis of rifamycin congeners (kanglemycins, Kangs) with potent in vivo and in vitro activity against the most common clinically relevant RifR mutations. Our structural and mechanistic analyses reveal the basis for Kang inhibition of RifR RNAP. Unlike Rifs, Kangs function through a mechanism that includes interfering with 5'-initiating substrate binding. Our results suggest that examining soil microbiomes for new analogues of clinically used antibiotics may uncover metabolites capable of circumventing clinically important resistance mechanisms.
Conflict of interest statement
S.F.B. founded and serves as a consultant for Lodo Therapeutics. The remaining authors declare no competing interests.
Figures
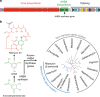



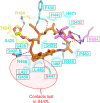
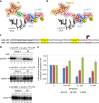

References
Publication types
MeSH terms
Substances
Grants and funding
- 1R35 GM122559/U.S. Department of Health & Human Services | National Institutes of Health (NIH)/International
- P41 GM103403/GM/NIGMS NIH HHS/United States
- R35 GM118130/GM/NIGMS NIH HHS/United States
- OPP1117928/Bill and Melinda Gates Foundation/International
- S10 RR027037/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

