Temperature affects the biology of Schmidtea mediterranea
- PMID: 30297872
- PMCID: PMC6175859
- DOI: 10.1038/s41598-018-33355-5
Temperature affects the biology of Schmidtea mediterranea
Abstract
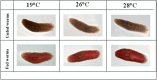
Studies of tissue regeneration and host-pathogen interactions using the model planarian Schmidtea mediterranea have been performed at an experimental temperature of 19 °C. S. mediterranea planarians exposed to 19 °C-32 °C were observed for survival, mobility, feeding and regeneration for three months and elimination of the Staphylococcus aureus pathogen over six days. S. mediterranea planarians died at 30 °C-32 °C after 18 days of observation but tolerated temperatures of 19 °C up to 28 °C with non-significant differences in mobility and feeding behavior. Genetic malleability tested by RNAi feeding was still efficient at 26 °C and 28 °C. Concerning the immune capacity of planarians, we reported an exacerbation of the immune response in worms infected by S. aureus at 26 °C and 28 °C. These observations suggest a temperature modulation of planarian stem cells and illustrate the importance of modulating experimental temperature when using planarians as model organisms to study regeneration and immune response.
Conflict of interest statement
The authors declare no competing interests.
Figures













References
-
- Benedict, J. E. et al. P. S. Pallas medicinae doctoris Miscellanea zoologica: quibus novae imprimis atque obscurae animalium species describuntur et observationibus iconibusque illustrantur, 10.5962/bhl.title.69851 (Apud Petrum van Cleef, 1766).
-
- Morgan TH. Experimental studies of the regeneration of Planaria maculata. Arch. für Entwickelungsmechanik der Org. 1898;7:364–397. doi: 10.1007/BF02161491. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

