Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma
- PMID: 30297909
- PMCID: PMC6481682
- DOI: 10.1038/s41591-018-0197-1
Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma
Erratum in
-
Author Correction: Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma.Nat Med. 2018 Dec;24(12):1941. doi: 10.1038/s41591-018-0251-z. Nat Med. 2018. PMID: 30361510
-
Publisher Correction: Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma.Nat Med. 2018 Dec;24(12):1942. doi: 10.1038/s41591-018-0252-y. Nat Med. 2018. PMID: 30361511
Abstract
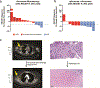
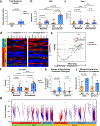
Preclinical studies suggest that treatment with neoadjuvant immune checkpoint blockade is associated with enhanced survival and antigen-specific T cell responses compared with adjuvant treatment1; however, optimal regimens have not been defined. Here we report results from a randomized phase 2 study of neoadjuvant nivolumab versus combined ipilimumab with nivolumab in 23 patients with high-risk resectable melanoma ( NCT02519322 ). RECIST overall response rates (ORR), pathologic complete response rates (pCR), treatment-related adverse events (trAEs) and immune correlates of response were assessed. Treatment with combined ipilimumab and nivolumab yielded high response rates (RECIST ORR 73%, pCR 45%) but substantial toxicity (73% grade 3 trAEs), whereas treatment with nivolumab monotherapy yielded modest responses (ORR 25%, pCR 25%) and low toxicity (8% grade 3 trAEs). Immune correlates of response were identified, demonstrating higher lymphoid infiltrates in responders to both therapies and a more clonal and diverse T cell infiltrate in responders to nivolumab monotherapy. These results describe the feasibility of neoadjuvant immune checkpoint blockade in melanoma and emphasize the need for additional studies to optimize treatment regimens and to validate putative biomarkers.
Conflict of interest statement
Figures


Comment in
-
New window of opportunity with ICIs in melanoma.Nat Rev Clin Oncol. 2018 Dec;15(12):723. doi: 10.1038/s41571-018-0124-x. Nat Rev Clin Oncol. 2018. PMID: 30410079 No abstract available.
References
-
- Liu J, et al. Improved Efficacy of Neoadjuvant Compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease. Cancer discovery 6, 1382–1399 (2016). - PubMed
-
- Eggermont AMM, Suciu S & Testori A Ipilimumab Adjuvant Therapy in Melanoma. The New England journal of medicine 376, 399 (2017). - PubMed
-
- Weber J, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. The New England journal of medicine 377, 1824–1835 (2017). - PubMed
-
- Eggermont AMM, et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. The New England journal of medicine 378, 1789–1801 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

