O-GlcNAc Modification During Pregnancy: Focus on Placental Environment
- PMID: 30298013
- PMCID: PMC6160872
- DOI: 10.3389/fphys.2018.01263
O-GlcNAc Modification During Pregnancy: Focus on Placental Environment
Abstract
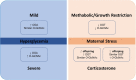
Successful placentation is a key event for fetal development, which commences following embryo implantation into the uterine wall, eliciting decidualization, placentation, and remodeling of blood vessels to provide physiological exchange between embryo-fetus and mother. Several signaling pathways are recruited to modulate such important processes and specific proteins that regulate placental function are a target for the glycosylation with O-linked β-N-acetylglucosamine (O-GlcNAc), or O-GlcNAcylation. This is a reversible post-translational modification on nuclear and cytoplasmic proteins, mainly controlled by O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA). O-GlcNAcylation has been implicated as a modulator of proteins, both in physiological and pathological conditions and, more recently, O-GlcNAc has also been shown to be an important modulator in placental tissue. In this mini-review, the interplay between O-GlcNAcylation of proteins and placental function will be addressed, discussing the possible implications of this post-translational modification through placental development and pregnancy.
Keywords: O-GlcNAc; placental dysfunction; placentation; post-translational modification; pregnancy.
Figures

Similar articles
-
Protein O-GlcNAcylation as a nutrient sensor signaling placental dysfunction in hypertensive pregnancy.Front Endocrinol (Lausanne). 2022 Dec 1;13:1032499. doi: 10.3389/fendo.2022.1032499. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36531508 Free PMC article.
-
O-GlcNAcylation in Hyperglycemic Pregnancies: Impact on Placental Function.Front Endocrinol (Lausanne). 2021 Jun 1;12:659733. doi: 10.3389/fendo.2021.659733. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34140929 Free PMC article. Review.
-
Yeast cells as an assay system for in vivo O-GlcNAc modification.Biochim Biophys Acta Gen Subj. 2017 May;1861(5 Pt A):1159-1167. doi: 10.1016/j.bbagen.2017.03.002. Epub 2017 Mar 2. Biochim Biophys Acta Gen Subj. 2017. PMID: 28263870
-
O-GlcNAcase Expression is Sensitive to Changes in O-GlcNAc Homeostasis.Front Endocrinol (Lausanne). 2014 Dec 1;5:206. doi: 10.3389/fendo.2014.00206. eCollection 2014. Front Endocrinol (Lausanne). 2014. PMID: 25520704 Free PMC article.
-
Functional Analysis of O-GlcNAcylation in Cancer Metastasis.Front Oncol. 2020 Oct 27;10:585288. doi: 10.3389/fonc.2020.585288. eCollection 2020. Front Oncol. 2020. PMID: 33194731 Free PMC article. Review.
Cited by
-
Environmental influences on placental programming and offspring outcomes following maternal immune activation.Brain Behav Immun. 2020 Jan;83:44-55. doi: 10.1016/j.bbi.2019.08.192. Epub 2019 Sep 4. Brain Behav Immun. 2020. PMID: 31493445 Free PMC article.
-
Disruption of O-Linked N-Acetylglucosamine Signaling in Placenta Induces Insulin Sensitivity in Female Offspring.Int J Mol Sci. 2021 Jun 28;22(13):6918. doi: 10.3390/ijms22136918. Int J Mol Sci. 2021. PMID: 34203166 Free PMC article.
-
A Metabolic Axis of Immune Intractability.Cancer Immunol Res. 2024 Mar 4;12(3):282-286. doi: 10.1158/2326-6066.CIR-23-0433. Cancer Immunol Res. 2024. PMID: 38126910 Free PMC article.
-
Protein O-GlcNAcylation as a nutrient sensor signaling placental dysfunction in hypertensive pregnancy.Front Endocrinol (Lausanne). 2022 Dec 1;13:1032499. doi: 10.3389/fendo.2022.1032499. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36531508 Free PMC article.
-
O-GlcNAcylation in Hyperglycemic Pregnancies: Impact on Placental Function.Front Endocrinol (Lausanne). 2021 Jun 1;12:659733. doi: 10.3389/fendo.2021.659733. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34140929 Free PMC article. Review.
References
-
- Briffa J. F., Hosseini S. S., Tran M., Moritz K. M., Cuffe J. S. M., Wlodek M. E. (2017). Maternal growth restriction and stress exposure in rats differentially alters expression of components of the placental glucocorticoid barrier and nutrient transporters. Placenta 59 30–38. 10.1016/j.placenta.2017.09.006 - DOI - PubMed
-
- Brown H. M., Green E. S., Tan T. C. Y., Gonzalez M. B., Rumbold A. R., Hull M. L., et al. (2018). Periconception onset diabetes is associated with embryopathy and fetal growth retardation, reproductive tract hyperglycosylation and impaired immune adaptation to pregnancy. Sci. Rep. 8:2114. 10.1038/s41598-018-19263-8 - DOI - PMC - PubMed
-
- Carosella E. D., Rouas-Freiss N., Paul P., Dausset J. (1999). HLA-G: a tolerance molecule from the major histocompatibility complex. Immunol. Today 20 60–62. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

